SIGNIFICANT RESULTS
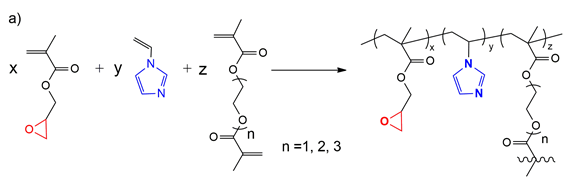
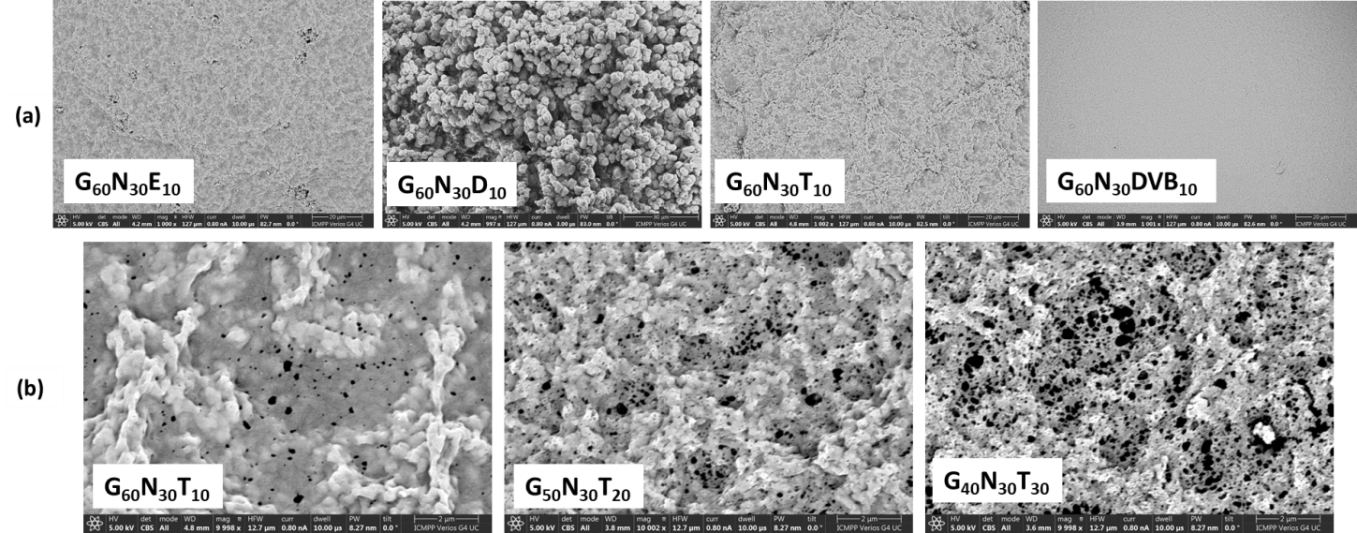
Optimization study in order to obtain porous microparticles with the best yield and specific surface area. These were obtained by suspension polymerization using the monomers N-vinylimidazole, glycidyl methacrylate and one of the crosslinkers: mono/di/triethyleneglycol dimethacrylate or divinylbenzene. Porous microparticles were synthesized under optimal conditions using toluene, n-butyl acetate, and a mixture of them 1:1 v/v as porogenic agents, obtaining spherical microparticles with average diameters between 200 and 350 µm. The yield and specific surface showed the following values: 91% and 48.32 m2/g (toluene), 93% and 61.7 m2/g (n-butyl acetate), respectively 90% and 51.7 m2/g (mixture toluene: n-butyl acetate (1:1 v/v)).
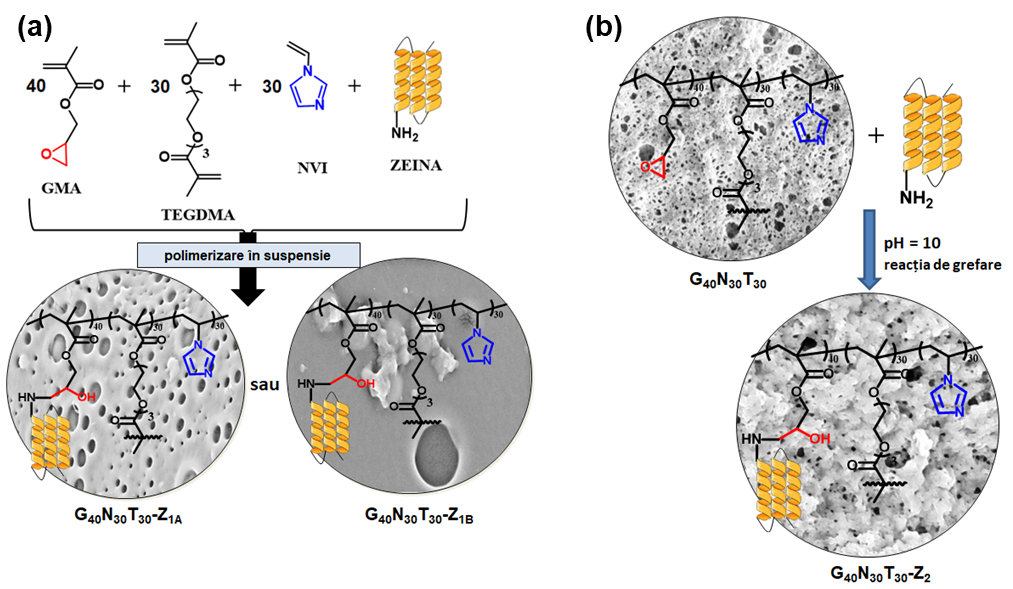
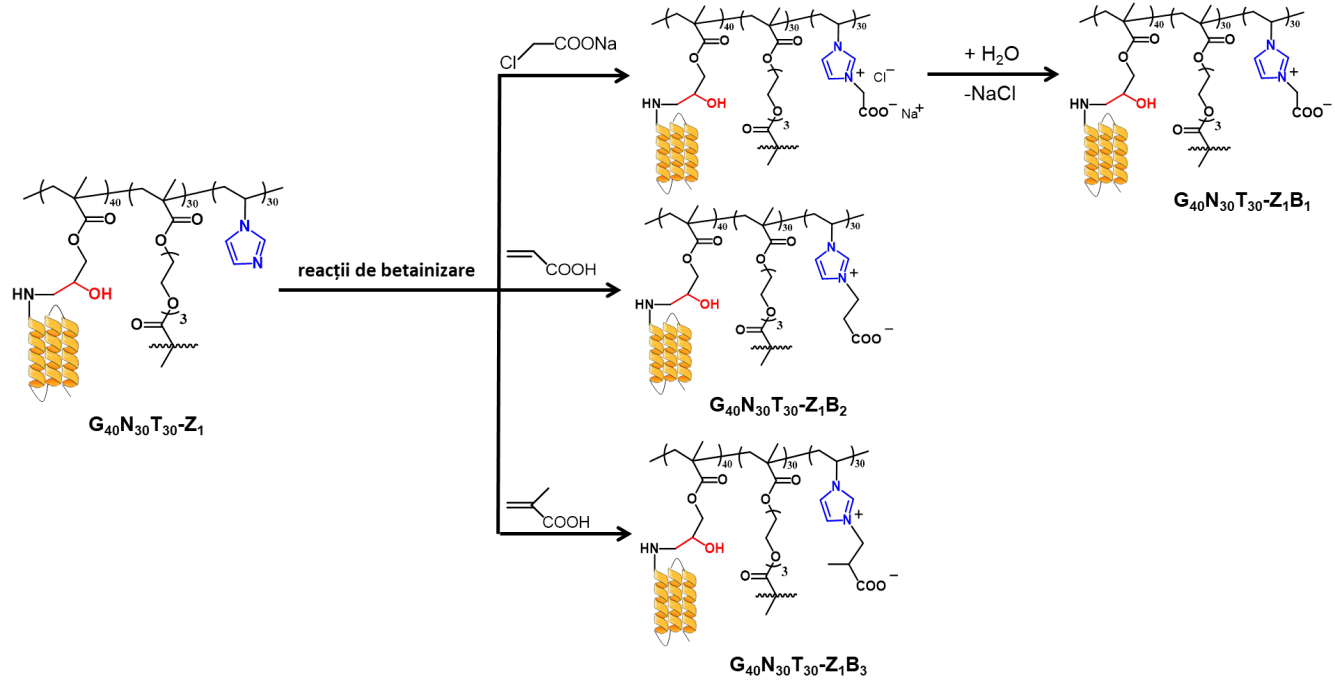
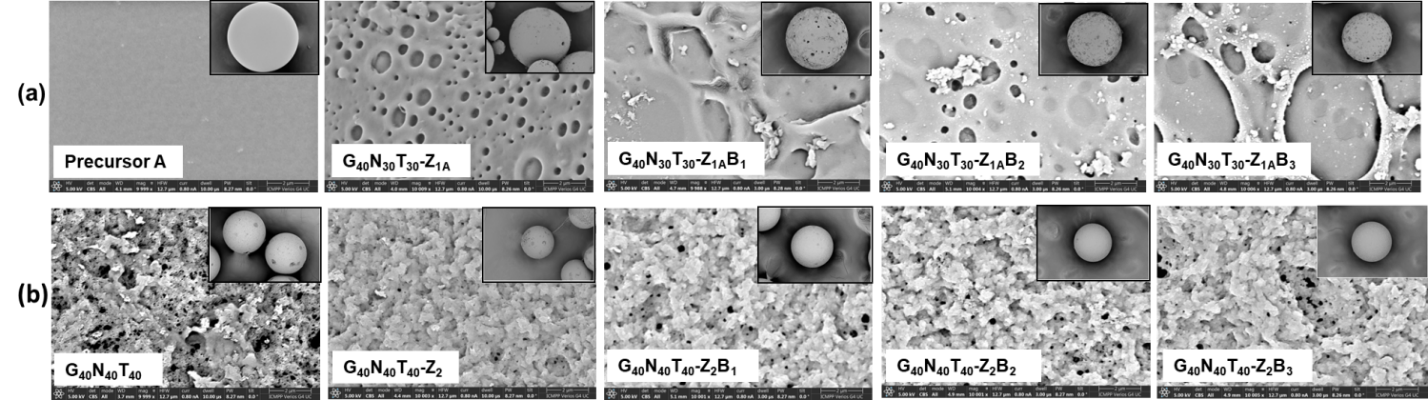
Hybrid microparticles with zein were synthesized by two methods: grafting by adding zein in the organic phase during suspension polymerization, or post-polymerization grafting at the level of porous microparticles. The two types of hybrid microparticles were subjected to the betainization process with sodium chloroacetate, acrylic acid and methacrylic acid.
The three types of microparticles were betainized using sodium chloroacetate, acrylic acid, methacrylic acid or 1,3-propanesultone. The highest degrees of betainization were obtained in the case of sodium chloroacetate.
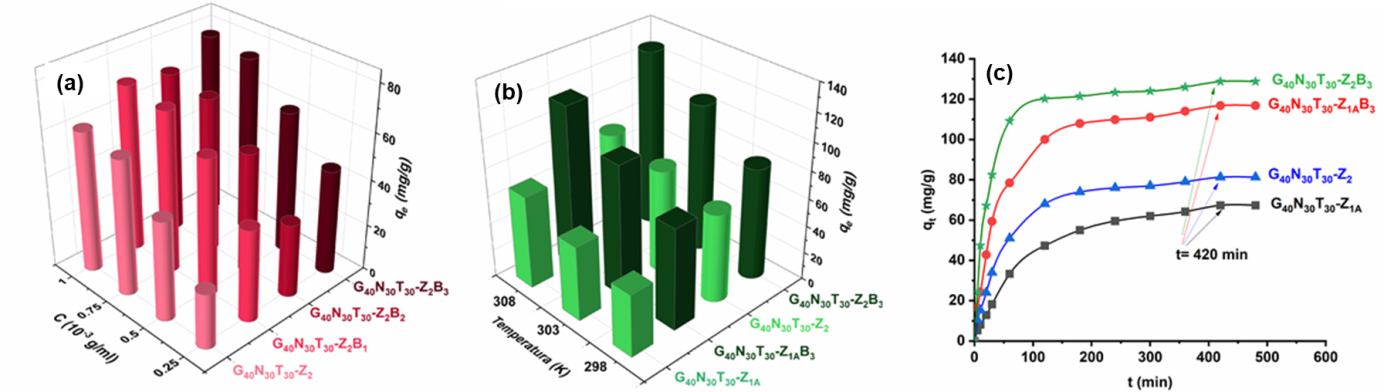
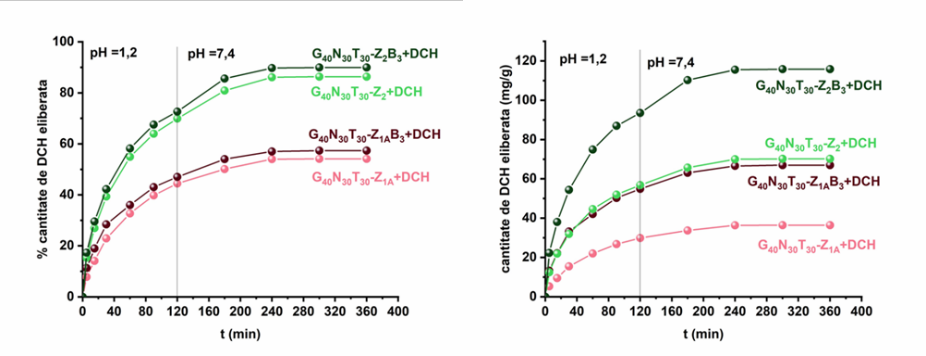
Loading/release tests of doxycycline hydrochloride (on zwitterionic microparticles) and tetracycline hydrochloride (on zwitterionic and hybrid zwitterionic microparticles) were performed. The study of release kinetics by applying different mathematical models highlighted the diffusional release mechanism of the two antibiotics.
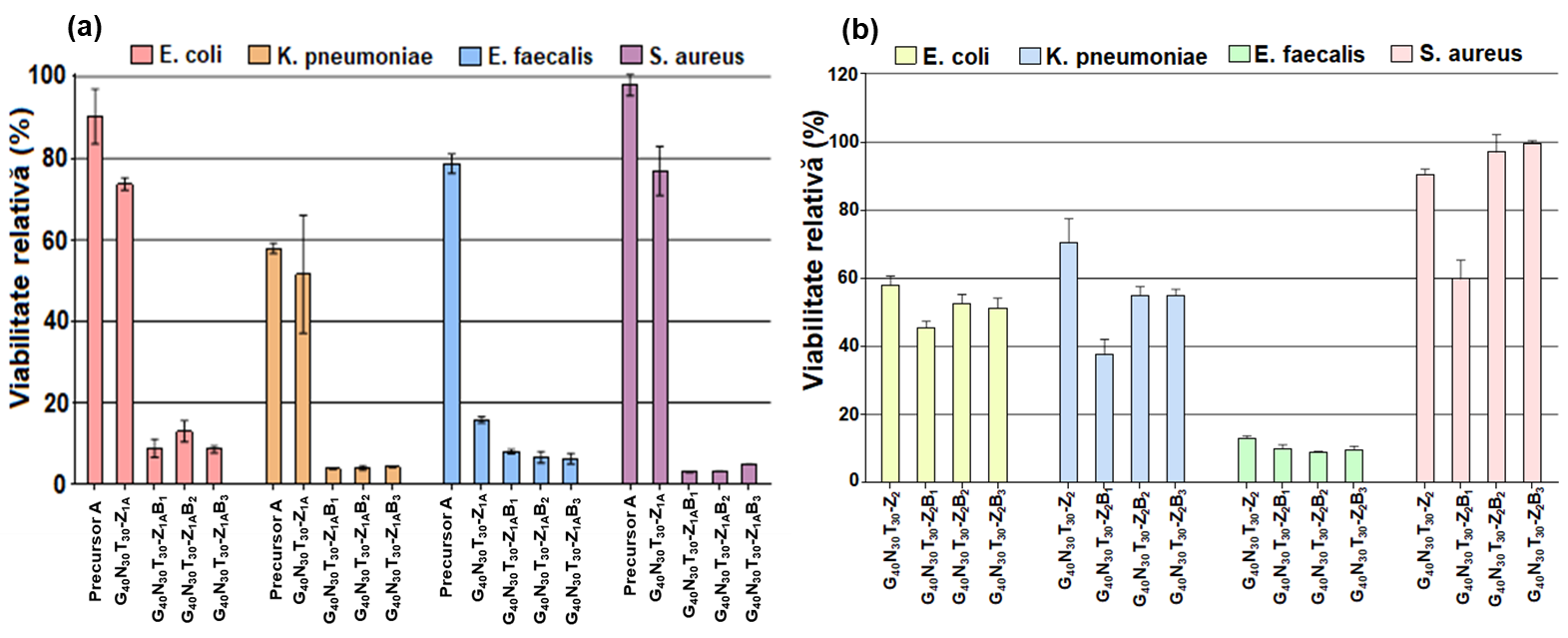
Antimicrobial activity of zwitterionic and hybrid zwitterionic microparticles was tested against strains of Staphylococcus aureus, Escherichia coli, Enterococcus faecalis and Klebsiella pneumoniae. The intrinsic antibacterial properties are enhanced by the presence of zwitterionic and zein groups. The best antibacterial activity is shown by zwitterionic microparticles based on (GNTZ)DMSO, which reduce the viability of all bacterial strains by more than 80%.
| 





