The main objective of this multidisciplinary project is to adopt a systematic research process in order to generate successful results, owing to SMPs potential as next generation organic electronic materials. We propose here the following three main objectives:
O1. The synthesis of a new family of host molecules
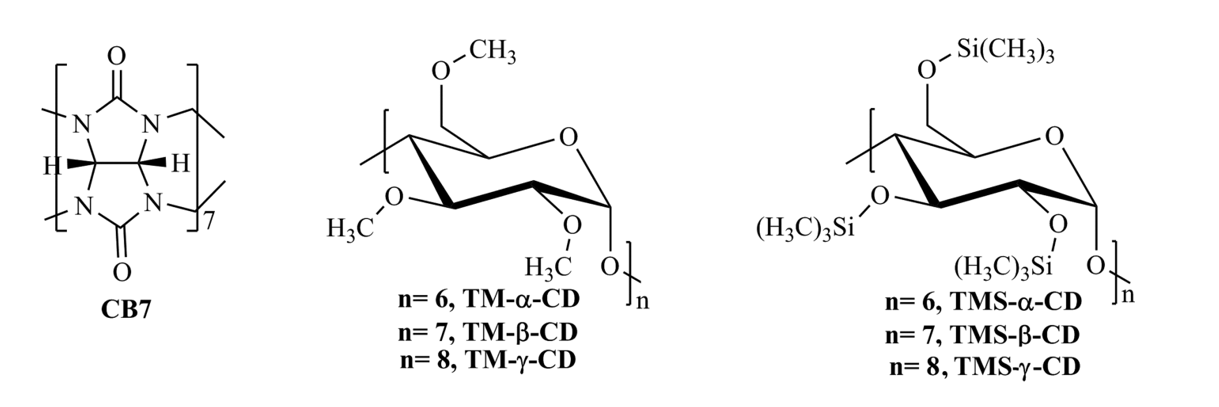
Figure 1. Chemical structures of host molecules.
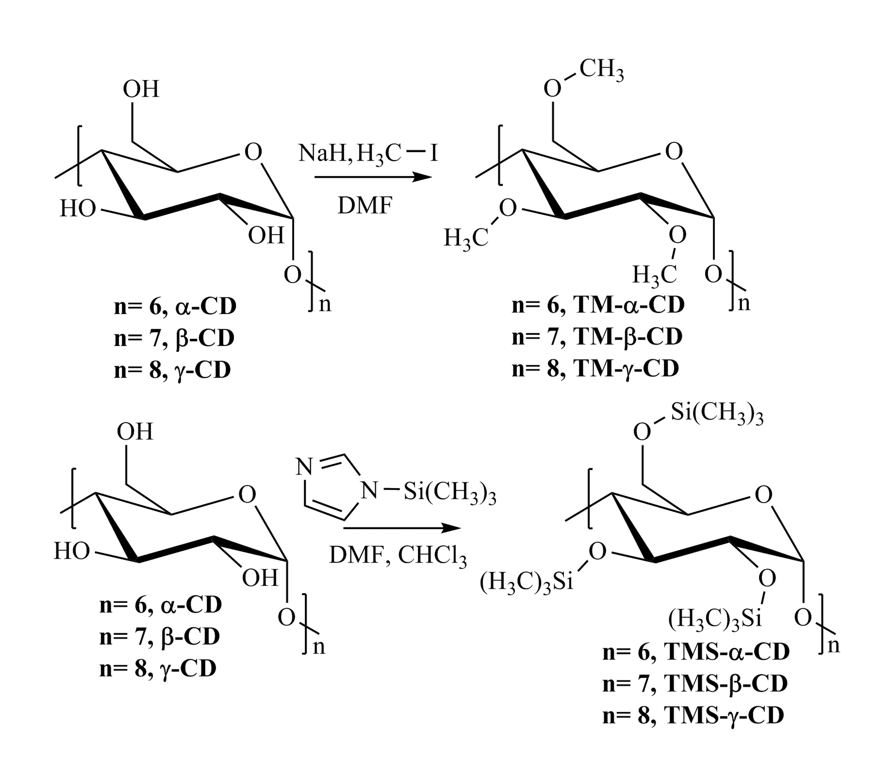
Scheme 1. Preparation of TM-CDs (up) and TMS-CDs (down)
Water-soluble Cucurbit[7]uril Privileged Macrocyclic Host Molecules
• Ultrahigh-affinity binding
• Biomimetic complexation-induced pKa shifts
• Lowest polarizability among condensed phases
• Highest symmetry, rigidity, and chemical inertness
• Role of cavitation energy as driving force
• Better fluorescence lifetimes
• Constrictive binding, in-situ click chemistry, molecular shuttles, drug delivery vehicles,
inner-phase reactions, etc.
O2. The synthesis of poly(3,4-ethylenedioxythiophene) (PEDOT) polypseudorotaxane (PS) and polyrotaxanes(PR) architectures
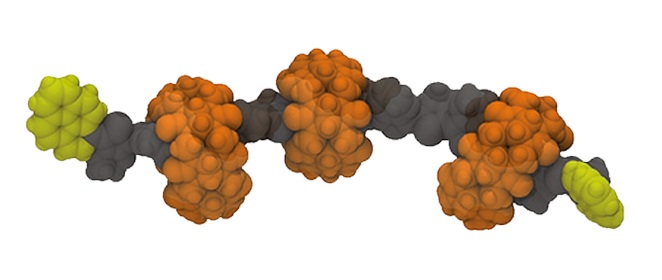
Figure 2. Schematic representation of PEDOT∙CB7-PR.
The synthetic strategy for the construction of CB7-based PEDOT-PPs and PEDOT-PRs involves as a first step the threading of EDOT into the CB7 cavity, through host-guest complexation, resulting in the formation of EDOT∙CB7 inclusion complex. This architecture represents the starting monomer for the subsequent polymerization process, a common strategy in the synthesis of PEDOT∙CB7-PS. The resulting PEDOT∙CB7-PS architecture will be then converted into its corresponding PEDOT∙CB7-PR by attachment of pyrene groups, as stoppers, at both ends of the PEDOT∙CB7-PS backbones to avoid the dethreading of CB7 in the purification steps. The uncomplexed PEDOT will also be synthesized by the coupling reaction of an aqueous EDOT dispersion with FeCl3 and, finally, the addition of pyrene as stoppers. Its photophysical and electronic properties will be systematically compared to those of the encapsulated PEDOT∙CB7-PS and PEDOT∙CB7-PR compounds.
O3. The synthesis of low band-gap poly(fluorene-thiophene-phenylene-azomethine) (PFTPA) PRs
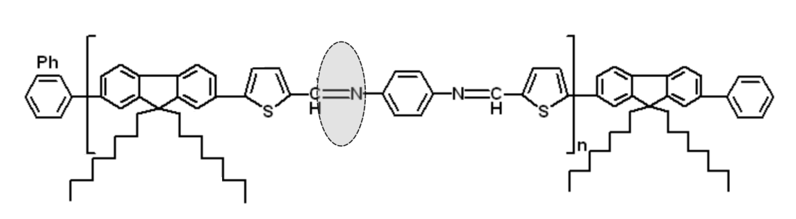
Figure 3. Chemical structures of PFTPA PRs.
The focus of our proposal is directed to the synthesis of new FTPAPRs as new low band-gap copolymers. Incorporation of the encapsulated thiophene-phenylene-azomethine (TPA) units in the PFs backbones could improve the oxidative stability of PFs copolymers. Another important aspect to be noted is that the rotaxination of TPA monomer is a promising approach to control the intermolecular aggregation between PFs chains that can improve the efficiency and stability of PSCs devices [1]. Thus, by Suzuki cross-coupling of the encapsulated TPA monomer with 9,9-dioctylfluorene-2,7-diboronic acid bis(1,3-propanediol) ester as bulky stopper and finally end-capping with bromobenzene, new PFTPA PRs will be obtained, Fig. 4. Additionally, we propose to investigate the Langmuir monolayer formations at the air-water interface of PFTPA PRs with TMS-CDs and TMe-CDs in the same manner with other PRs recently reported by our group [2]. The Langmuir monolayer formations are essential for the exploitation into various electronic devices.
References
[1]. a) A. Farcas, A.-M. Resmerita, Supramolecular chemistry: synthesis and photophysical characteristics of conjugated polyrotaxanes, in: Z. Wang (Ed), Encycl. Phys. Org. Chem., Vol. 3, John Wiley& Sons, Hoboken, USA, 2017, pp. 2543-2582; b) A. Farcas, P.-H. Aubert, Electrochemical studies of conjugated polyrotaxanes and their unthreaded analogs, in: Z. Wang (Ed.), Encycl. Phys. Org. Chem., Vol. 3, John Wiley& Sons, Hoboken, USA, 2017, pp. 2583–2619.
[2]. A. El Haitami, A.-M. Resmerita, O. Fichet, S. Cantin, P.-H. Aubert, A. Farcas, Synthesis, photophysics and Langmuir films of polyfluorene/permodified cyclodextrin polyrotaxanes, Langmuir Langmuir 2021, 37, 11406−11413.
|






