IMPLEMENTATION STAGE 1/2025
Design, preparation, and characterization of customized DESs (deep eutectic solvents) and polymeric components: dialdehyde cellulose nanofibers (DAC) and the polyaminic analogue of polyacrylonitrile (PANH)
SUMMARY
• A new eutectic solvent required for the subsequent preparation of the layered hydrogel was designed and produced. This solvent is composed of a mixture of choline chloride (ChCl) and 4-carboxyphenylboronic acid (4-CPhB). Choline chloride has a melting point of 302 °C, while 4-carboxyphenylboronic acid has a melting point of 220 °C. Remarkably, through a laborious methodology, we succeeded in obtaining a physical mixture, in various molar ratios between these two compounds, that is liquid at room temperature and can act as an efficient solvent for a wide range of compounds insoluble in common organic solvents.
• The new DES solvent was analyzed, demonstrating its stability. The methods used to evaluate the formation and stability of the DES were: FTIR spectroscopy, NMR, thermal analysis techniques, and microscopic methods. The influence of certain parameters—such as the molar ratios between the two components, water, ultrasonication, lyophilization, and metal salts—on the DES formation process was also studied.
• Cellulose nanofibers incorporating aldehyde groups on their surface were successfully synthesized through a reaction protocol involving the oxidation of cellulose with sodium periodate (NaIO4). The oxidized cellulose samples were characterized spectrally and optically, and the aldehyde group content introduced into the cellulose structure after oxidation was also determined.
• All indicators provided in the “project implementation plan” were achieved: 2 scientific papers submitted for publication (6 scientific papers were actually published), 4 presentations at international scientific conferences, including 3 oral presentations and one poster (actually achieved: 4 invited lectures at international conferences delivered by the project director, 2 oral presentations, and 7 posters).
Act. 1.1 – Preparation of DESs by Different Methods (O1 – WP2 (ACT. 2.1))
DESs represent a class of solvents obtained by combining two or more solid components which, in well-defined molar ratios, form a mixture with a melting point much lower than that of any individual component. This phenomenon is known as the eutectic effect and is mainly due to intermolecular interactions (hydrogen bonds) that arise between the involved species. Such mixtures can form liquids that are stable at room temperature, even if each component is solid in its pure form. Because of these properties, eutectic solvents are considered sustainable alternatives to traditional organic solvents and are compatible with the principles of green chemistry.
The formation of a DES typically involves the association of two solid components—one acting as a hydrogen bond acceptor (HBA) and the other as a hydrogen bond donor (HBD). Upon mixing, a rearrangement of intermolecular interactions occurs and the total free energy of the system decreases. Thus, the hydrogen bonds between the HBA and HBD disrupt the ionic or van der Waals interactions within the solid lattices, leading to a significant decrease in the melting point of the mixture compared to the pure components. A classic example is the mixture of choline chloride and urea, in a 1:2 molar ratio, which forms a liquid stable at room temperature, although choline chloride and urea have melting points of 302 °C and 133 °C, respectively.
Within Act. 1.1, several tests were carried out for the preparation of eutectic solvents, particularly those based on choline chloride (ChCl) and 4-carboxyphenylboronic acid (4-CPhB).
Influence of lyophilization on the DES solvent formation process
When testing the solubility of the two components, it was observed that ChCl is soluble in water, while 4-CPhB is soluble in DMF. Thus, for the preparation of the DES solvent, in a first step two solutions were prepared: a 5% ChCl solution in water and a 5% 4-CPhB solution in DMF. Then, mixtures of these solutions were prepared in different ratios (Table 1).
Table 1. The molar ratios used to test the preparation of the ChCl:4-CPhB eutectic mixture by lyophilization.
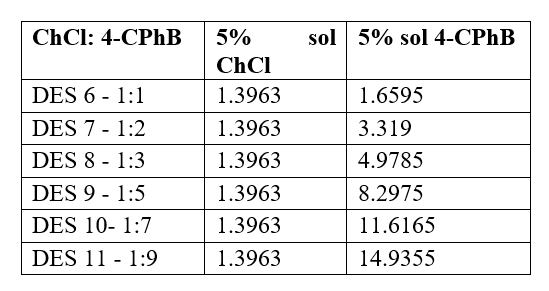
After mixing the two solutions in the established molar ratios (Table 1), the samples were immediately frozen in liquid nitrogen and lyophilized for 48 h, Fig. 1.

Fig. 1. Appearance of the ChCl:4-CPhB DES mixtures in different molar ratios after lyophilization.(OBS. Depending on the molar ratio used, the samples behaved differently: sample DES 6 (1:1) has a homogeneous, transparent appearance and the solution is slightly viscous; samples DES 7 (1:2) and DES 8 (1:3) remained solid after lyophilization (the vials broke during freezing in liquid nitrogen); samples DES 9 (1:5) and DES 10 (1:7) are slightly homogeneous mixtures but opaque; sample DES 11 (1:9) has a pasty appearance (and in this case the vial cracked during freezing in liquid nitrogen).
Act. 1.2 – Analysis and Characterization of DESs (O1 – WP2 (Act. 2.2.))
Fourier Transform Infrared Spectroscopy (FT-IR)
To investigate the intermolecular interactions between the two components and to highlight the structural characteristics of the obtained eutectic mixture, FT-IR spectra were recorded. By comparatively analyzing the spectra of the starting compounds and the DES solvent, several spectral changes induced by the interaction between ChCl and 4-CPhB were observed, Fig. 2.
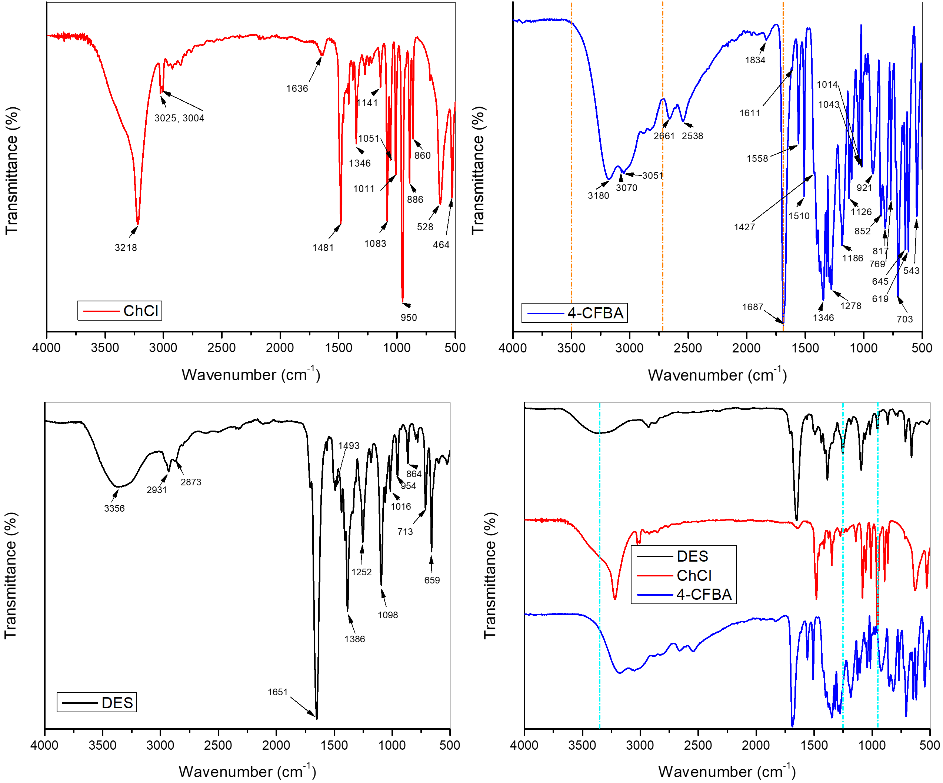
Fig. 2. FTIR spectra of the starting compounds ChCl, 4-CPhB, of the obtained eutectic solvent (DES), and the overlaid spectra of the starting compounds and the obtained DES.
Act. 1.3 – Selective Oxidation of Cellulose with Sodium Periodate (O2–WP3 (Act. 3.1.))
For the functionalization of polysaccharides, oxidation generally represents the main strategy. For the selective oxidation of cellulose, sodium periodate was used. The oxidation of cellulose in the presence of sodium periodate occurs at the level of the secondary hydroxyl groups, leading to the formation of two aldehyde groups (2,3-dialdehyde cellulose), with the reaction being accompanied by the cleavage of the C–C bond between positions C2 and C3 (Scheme 1). For the oxidation of cellulose in the presence of sodium periodate, several types of cellulose with different degrees of polymerization were tested. The oxidation of cellulose in the presence of sodium periodate was carried out according to the following protocol: 10 g of cellulose were combined with 10 g of NaIO₄ (4.6 mM/g cellulose). The oxidation reaction was conducted in the absence of light, at a pH of 4–5, for 4 hours.
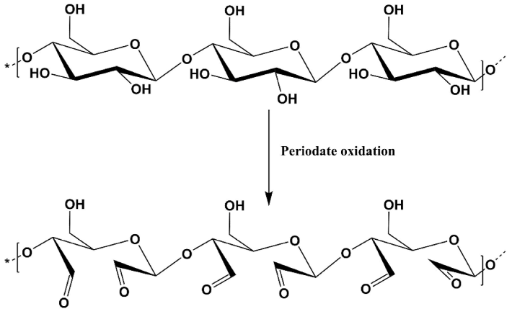
Scheme 1. Mechanism of selective oxidation of cellulose in the presence of sodium periodate.
Act. 1.4 – Characterization of oxidized products (O2 – WP3 (Act. 3.2.))
Fourier Transform Infrared Spectroscopy (FT-IR)
For the characterization of cellulose before and after oxidation, the FTIR technique was used. In the FTIR spectrum of the control cellulose, a broad band is observed in the 3200–3400 cm⁻¹ region, attributed to ν(OH) groups; a band around 2890–2920 cm⁻¹, specific to ν(C–H) vibrations of the methylene and methine groups of the glucopyranose chain. At 1644 cm⁻¹, a band associated with the symmetric deformation vibrations of water molecules is observed. In the 1200–900 cm⁻¹ region, a set of bands characteristic of the asymmetric deformation vibrations of (C–O–C) groups, the stretching vibrations of (C–O) groups, the deformation vibrations of CH2OH and CHOH groups, and pyranose rings is observed, confirming the cellulose structure. After oxidation, in the FTIR spectrum of Cox, the presence of an additional band at 1725 cm⁻¹ is observed; this band is specific to aldehyde groups ν(C=O). Furthermore, the intensity of the band in the 3200–3400 cm⁻¹ region slightly decreases, as a result of the partial conversion of hydroxyl groups to aldehyde groups during the oxidation process.
Scanning Electron Microscopy (SEM)
The morphology of the cellulose samples, before and after oxidation, was investigated using scanning electron microscopy (SEM), Figure 3. The SEM image of the oxidized sample shows a visibly modified surface, much smoother and more compact. The oxidized cellulose exhibits a loss of the initial fibrous appearance, with a reduction in roughness and cracks, the surface becoming more continuous as a result of microfibril disruption (breaking of the bonds between C2 and C3) during the oxidation process. A noticeable “collapse” of the structure is also observed, with the oxidized material becoming denser.
Fig. 3. SEM images of the cellulose sample before and after oxidation.
Act. 1.5 – Preparation and Characterization of Nanofibrils (O2 – WP3 (Act. 3.3.))
For the extraction of nanocellulose, the DES mixture obtained and characterized in Act. 1.1 and Act. 1.2 was used. The cellulose grade used for testing was α-cellulose with a Cr.I. = 68 and a DP = 950.
Extraction protocol: 0.6 g of cellulose was added to 10 mL of DES. The resulting suspension was stirred for 3 days at 70 °C to allow swelling/activation, fragmentation, and disaggregation of the cellulose structure under the action of the DES. After 3 days, a mixture of H₂O/DMF (approximately 60 mL water / 90 mL DMF) was added to the obtained suspension. The resulting mixture was filtered and washed on the filter with the H₂O/DMF solvent mixture to remove any residual DES. After washing, the obtained nanocellulose was recovered from the filter and precipitated in acetone (excess acetone). The precipitated suspension was stored in the freezer for 24 hours, then filtered again, and the resulting solid was collected and redispersed in water (Fig. 4).

Fig. 4. Morphology of the nanocellulose (Cell_DES) extracted using the ChCl:4CPhB (1:1) eutectic mixture.
COGNITIVE AND SOCIO-ECONOMIC IMPACT
This project generates significant cognitive and socio-economic impact by advancing green solvent chemistry and bio-based nanomaterials engineering, while enabling sustainable, high-value applications in healthcare, bioelectronics, and environmental technologies. Scientifically, it expands the design space of deep eutectic solvents (DESs) through the development of a novel choline chloride–4-carboxyphenylboronic acid system capable of dissolving and activating cellulose under mild conditions. The project establishes new mechanistic understanding of hydrogen-bond-driven eutectic formation, solvent–polymer interactions, and selective cellulose oxidation, together with reproducible protocols for nanofibrillation and functionalization. These methodological advances provide transferable tools for sustainable polymer processing and set the foundation for next-generation hydrogels, films, and nanocellulose architectures.
The work strengthens Europe’s knowledge base by integrating green chemistry, polymer science, nanotechnology, and biomedical materials into a unified platform. Through high-quality publications, invited lectures, and international dissemination, the project contributes to rapid knowledge diffusion and builds human capital by training early-career researchers in advanced spectroscopy, materials characterization, and sustainable manufacturing approaches. This interdisciplinary skill development enhances Europe’s research excellence and innovation readiness in advanced materials.
From a socio-economic perspective, the project directly supports the EU Green Deal and circular bioeconomy objectives by replacing hazardous organic solvents with low-toxicity DESs and by valorizing renewable cellulose feedstocks. The resulting processes reduce environmental impact, improve workplace safety, and lower waste management and regulatory costs. The developed technologies have clear industrial relevance across multiple sectors, including biomedical hydrogels and wound care materials, drug delivery systems, flexible and wearable bioelectronics, antimicrobial and protective coatings, fuel-cell membranes, and environmental remediation materials. These applications open pathways toward scalable production of bio-based alternatives to petrochemical materials, strengthening European competitiveness and strategic autonomy in sustainable advanced materials.
The expected downstream effects include new intellectual property, opportunities for spin-offs and SME engagement, technology transfer to industry, and the creation of high-skill jobs. In healthcare contexts, biocompatible and functional cellulose-based materials may contribute to improved treatment outcomes, reduced infection rates, and lower system costs through safer and more affordable devices. Regionally, the project enhances innovation capacity, attracts collaborations and funding, and consolidates leadership in green materials research.
Overall, the project delivers high cognitive value through new scientific principles and training, and strong socio-economic benefits through sustainable manufacturing routes, market-ready technologies, and contributions to environmental protection, public health, and economic growth. | 





