Stage 1/2025 - Preparation of cellulose precursors of the nanofiber type, ZnO nanoparticles, and aerogels for the support layer (lower layer)
Activity 1.1. - Preparation of cellulose nanofibers (CNF) with carboxyl groups on the surface (Objective 1 – WP2)
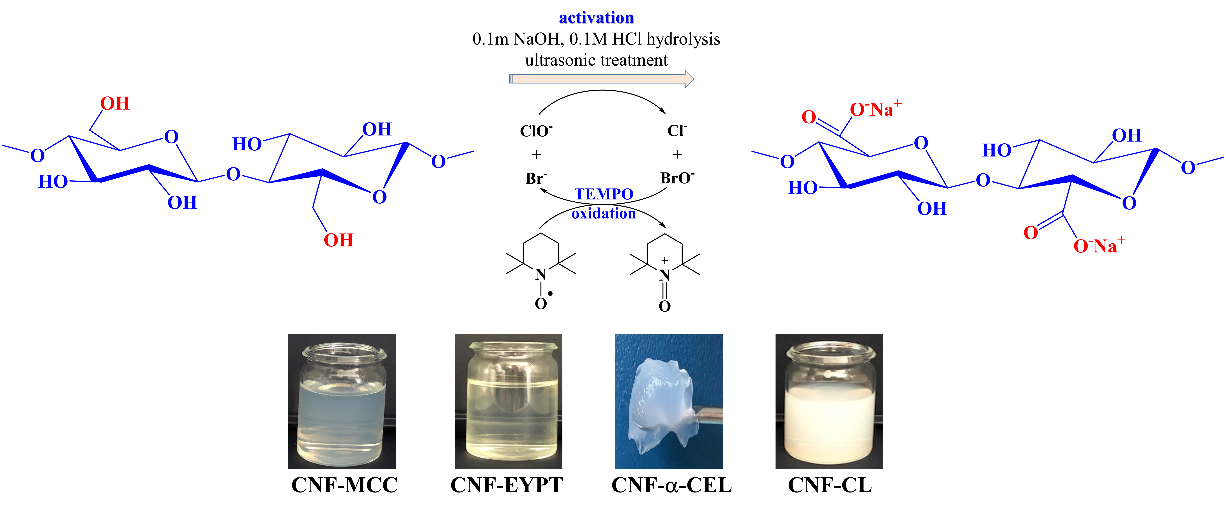
- Oxidized cellulose nanofibers were successfully obtained using the TEMPO/NaClO/NaBr protocol, starting from various cellulose sources (CL, MCC, α-CEL, TEG1-2, EYPT) after physico-chemical activation, resulting in CNF with different carboxyl group contents and high colloidal stability.
Figure 1. Schematic representation of the CNF extraction process.
- The obtained products were characterized structurally, morphologically, and physicochemically by FTIR, XRD, SEM, TEM, conductometric titration, and zeta potential measurements, confirming selective oxidation, increased crystallinity, and the obtaining of nanofibers with dimensions in the nanometer range (diameter 48–90 nm, length 108–445 nm); commercial CNCs were used as comparison materials.
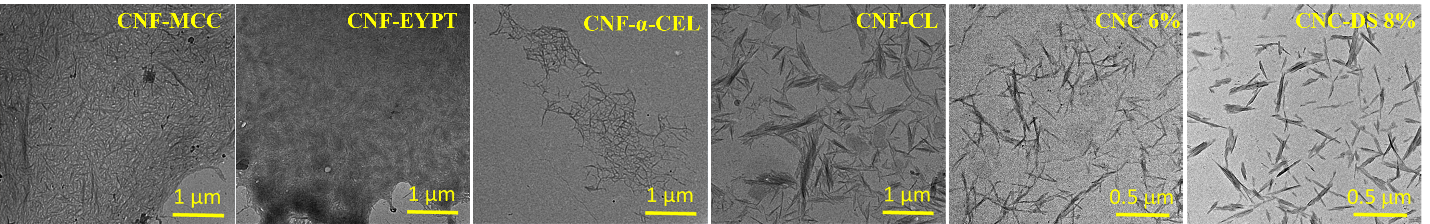
Figure 2. TEM images of CNF and CNC samples.
Activity 1.2. - Obtaining unmodified ZnO nanoparticles (NPs) with different morphologies and transforming them into cryo-aerogel (Objective 2 – WP3)
-
Three types of ZnO nanoparticles were synthesized by the coprecipitation method, using surfactants with different charges (nonionic, anionic, and cationic) to control morphology and size, obtaining nanoparticles with a wurtzite crystal structure and average crystal sizes between 21–31 nm.
-
Characterization by FTIR, XRD, SEM, UV-Vis confirmed the obtaining of pure ZnO, with optical properties dependent on the nature of the surfactant, and DRS (Eg = 3.10–3.16 eV) and photoluminescence studies indicate improved photocatalytic potential.
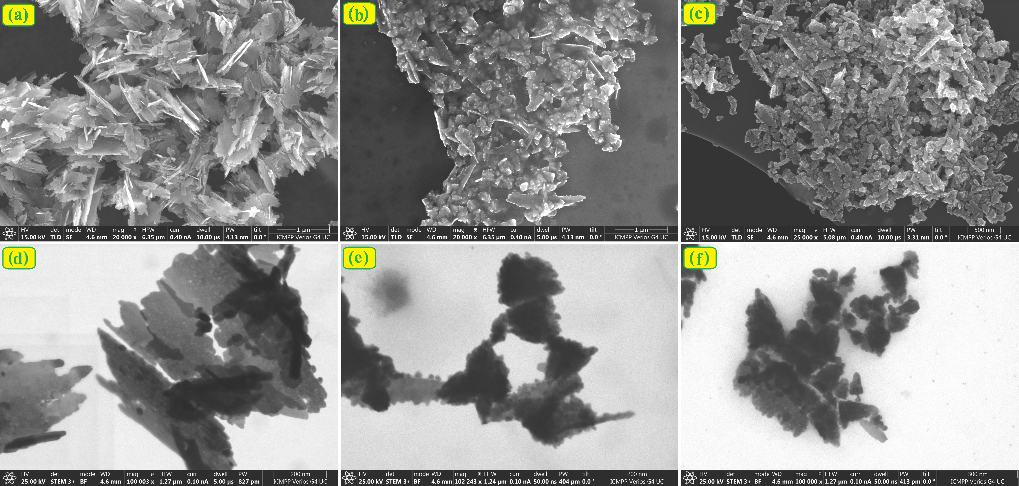
Figure 3. SEM (a, b, c) and STEM (d, e, f) micrographs of ZnO powders (ZnO-1 – a, d; ZnO-2 – b, e; ZnO-3 – c, f) synthesized during the stage.
- The nanoparticles were subsequently transformed into cryo-aerogel by rapid freezing and freeze-drying, obtaining structures with improved dispersion and reduced density.
Activity 1.3. - Preparation of the support layer (lower layer) of the floatable system (Objective 3 – WP4)
-
The hydrophilic polymer PVTMS was synthesized by radical polymerization under optimized conditions (110 °C, 48 h, 10 mol% DTBP), the structure being confirmed by FTIR and 1H NMR, and the molecular weight determined by GPC (Mw = 8.5 × 103 g/mol).
-
Hydrophilic CNF–PVTMS aerogels were prepared by freeze-drying and thermal polycondensation, obtaining ultra-light, floatable structures with densities between 15.7–87.9 mg/cm3 and controllable porosity by varying the CNF:PVTMS ratio.
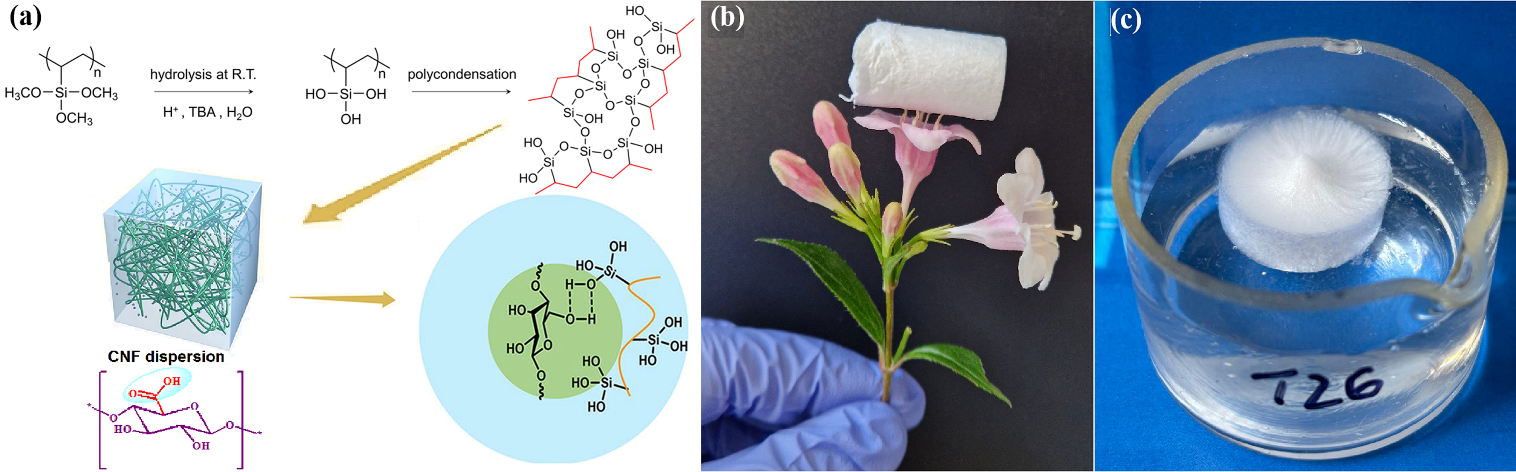
Figure 4. Schematic of the preparation method for nanocellulose-based aerogels (a) and photographs of the obtained samples, highlighting their ultralightweight character (b) and floatability (c).
- FTIR and SEM analyses evidenced the formation of covalent bonds and CNF–PVTMS hydrogen-bonding interactions, as well as macroporous tubular/channel-like architectures (A-8–A-12) that favor reactant transport toward the catalytic layer; these samples were selected as optimal for the next stage.
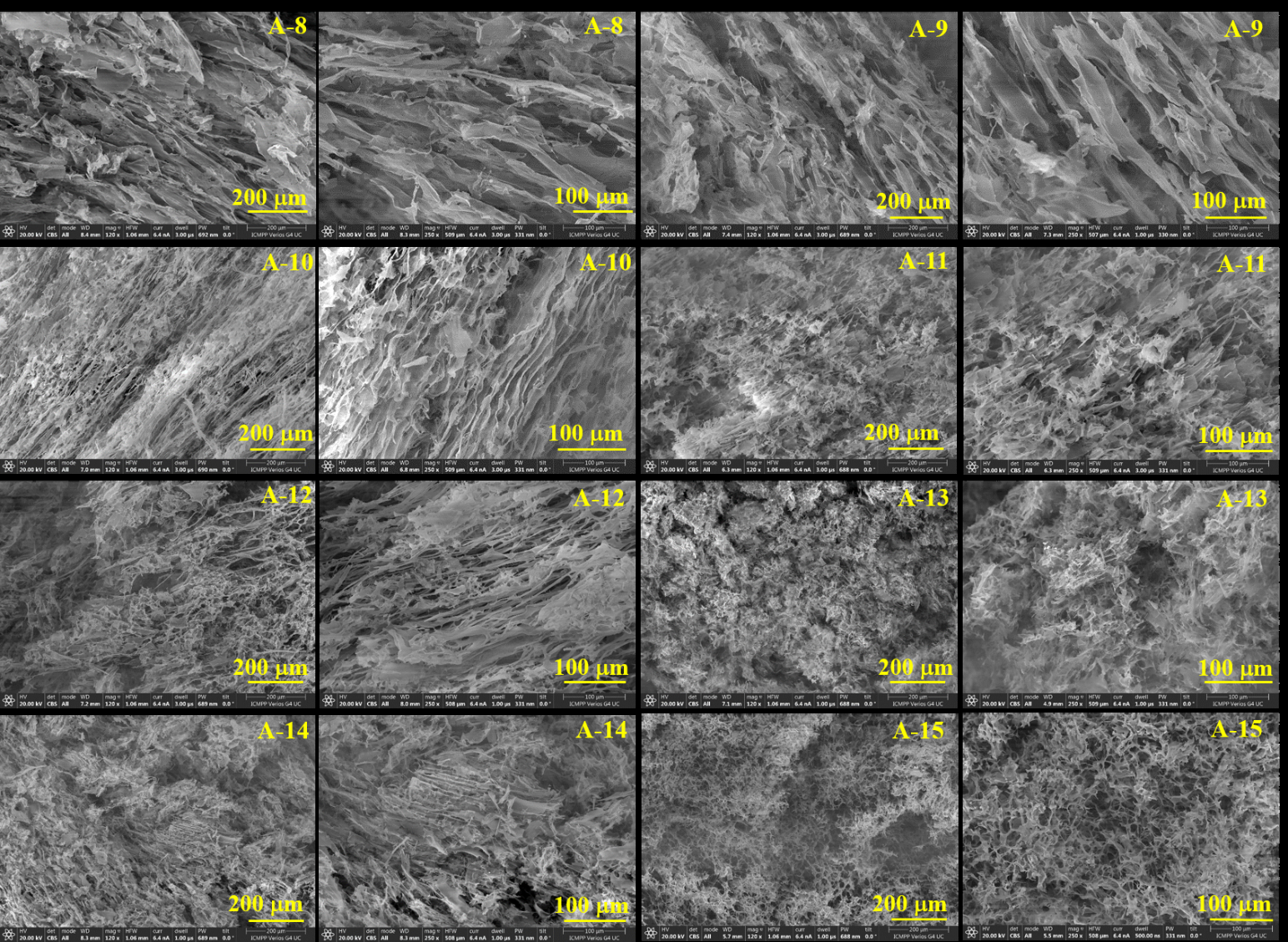
Figure 5. 15 SEM images at 120x and 250x magnification of aerogels A-8 – A-15.
- All performance indicators in the project implementation plan were achieved: 2 scientific papers published in Q1 journals in materials science/biomaterials or engineering/chemistry (exceeded with 3 publications: 2 in Q1 journals and 1 in a Q2 journal), and 4 international scientific conference presentations (exceeded with 8 contributions, oral and poster).
|






