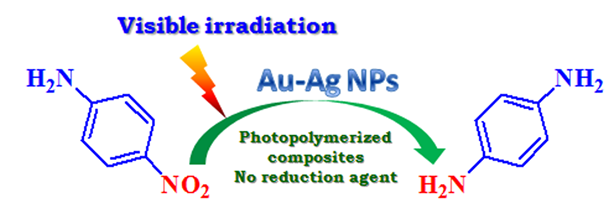
Photocrosslinked hybrid composites containing Ag, Au or Au-Ag NPs as visible light triggered photocatalysts for degradation/reduction of aromatic nitroderivatives
Flexible free-standing nanocomposite films embedding Ag, Au or Au-Ag nanoparticles were successfully synthesized by photocrosslinking of organic matrix in tandem with photogeneration of noble metal nanoparticles (e.g. Ag, Au or Au-Ag NPs). The photocatalytic activity of the obtained catalysts was evaluated following the photodegradation of 4-nitroaniline (under ambient conditions and visible irradiation) or the photoreduction of 4-nitroaniline to p-phenylendiamine (in the presence of NaBH4). Further modification of the organic matrix by the inclusion of 3-acrylamidophenylboronic acid monomer, allowed us to prepare a catalyst with the hydrogen donor compound included in the polymer film. The photoreduction of 4-nitroaniline to p-phenylenediamine occurred in the presence of the new photocatalyst without the addition of a reducing agent in the reaction mixture.
- Synthesis of acrylic and urethane (silyl-urethane) acrylic monomers used to the preparation of copolymers, photopolymers, block copolymers and polymer networks
- Studies of photochemical behavior in polymer solutions and films under UV/vis or laser pulses (femtoseconds) exposure
- Production of polymer-inorganic nanoparticles hybrid composites (Au, Ag, ZnO, TiO2, grafene, etc.)
- Testing urethane macromers and some modified acrylic copolymers in obtaining dental composites and adhesives, thin polymer films, fluorescence sensors
- Achievement of polymer scaffolds for cell growth using two-photon polymerization (2PP) of some urethane (silyl-urethane) monomers and macromers
- Testing hybrid composites in photocatalysis, antimicrobial coatings.
European Polymer Journal 121 (2019) 109289
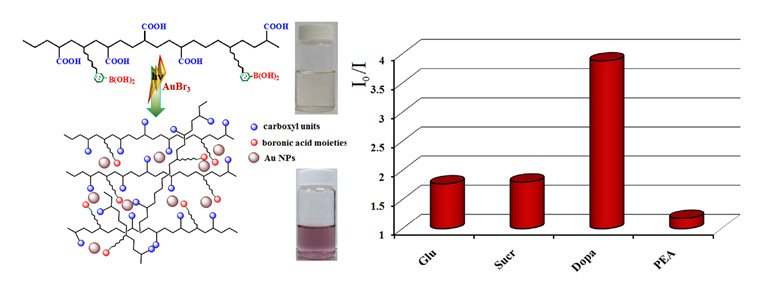
Fluorescent polymeric sensors containing boronic acid derivatives for sugars and dopamine detection. Sensing characteristics enhancement by Au NPs
Boronic acid copolymers were successfully obtained by free radical polymerization process (between 3-acrylamidophenylboronic acid/4-vinylphenylboronic acid with N-acryloyl-L-alanine and vinyl acetate) in order to investigate the role of the boronic acids in the fluorescence response mechanism.
Such copolymers were tested as fluorescent sensors at physiological pH for detection of diols, namely sugars (glucose, sucrose) and catecholamines (dopamine). The sensitivity for dopamine (Cmin = 4 × 10−5 M) was higher than for the sugar derivatives (Cmin = 6 × 10−4 M) and the fluorescence quenching of the copolymers mainly occurred through dynamic processes. An enhancement of sensor sensitivity was performed by the in situ photogeneration of gold nanoparticles (Au NPs) into copolymer solution, upon exposure to UV irradiation, when minimal dopamine concentration was detected (4 × 10−6 M).
Sensors and Actuators B 253 (2017) 987–998
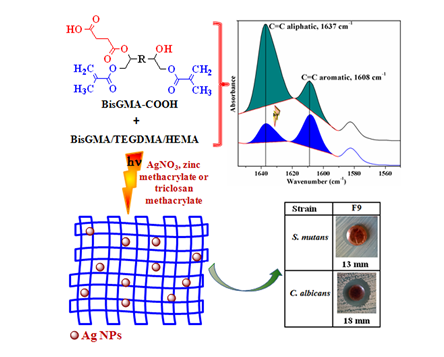
New acid BisGMA analogs for dental adhesive applications with antimicrobial activity
Bisphenol A glycerolate dimethacrylate (BisGMA) analogs with reduced viscosity were successfully achieved in order to be used in various formulations of the dental adhesives containing biocidal components. Thus, a series of low-viscosity BisGMA derivatives (η: 39–12 Pa s) modified carboxylic acid units were synthesized and characterized. Hydrogen bonding interactions, the photopolymerization behaviour and implicitly the conversion degree (DC), the water effects on the photocrosslinked networks and the flexural strength/modulus were investigated by specific analyses. The adhesive penetration into the dentin surface was surveyed by SEM analysis, and the antimicrobial activity triggered by the incorporation of 0.5 wt% AgNO3, 10 wt% zinc methacrylate or 1 wt% triclosan methacrylate in selected adhesive formulations on the growth of Streptococcus mutans and Candida albicans strains was also evidenced. The experimental formulations based on carboxyl-functionalized BisGMA exhibit a similar or even improved behaviour over control sample, suggesting their potential applicability as antimicrobial dental adhesives.
Dental Materials 32 (2016) e314–e326 |






