RAPORT ETAPA 1/2021
Synthesis and characterization of cellulose-based derivatives: the future matrix for N-heterocyclic compounds incorporation
• Selective oxidation reactions of the primary OH groups for different cellulosic sorts were performed by processes that are developed in our laboratory, the structure of oxidized materials being confirmed by FTIR, 13C-NMR and X rays, morphology by scanning electron microscopy (SEM). These materials represent the basis that will be used later for the preparation of proton conducting membranes in the next steps.
• The introduction of a considerably larger number of carboxylic groups into the structural unit of cellulose was made possible by its functionalization with citric acid, a reaction performed at high temperature.
• An ecological alternative was presented for the production of nanocrystalline cellulose from agricultural waste, namely hemp stems resulting from the harvesting process.
• All the indicators provided in the “project implementation plan” were completed: 3 papers submitted for publication (completed 5), 2 oral presentations and 4 posters (completed 3 oral presentations and 4 posters).
Activity 1.1. Preparation and characterization of carboxylated cellulosic substrates by the TEMPO oxidation method
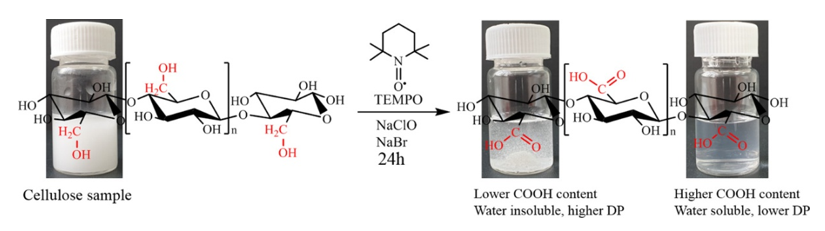
Fig. 1. Pattern of cellulose oxidation reaction in the presence of TEMPO, NaClO, NaBr
The oxidation products showed almost homogeneous structures of poly-β-D-(1→4)-sodium glucuronate or sodium salt of celuronic acid (CUA) consisting only of D-glucuronosyl units. Therefore, the primary hydroxyl groups in the cellulose structure can be converted entirely and selectively into carboxylate groups (carboxylate groups in the form of sodium salt) by TEMPO-mediated oxidation. It has been confirmed that C6-carboxylic groups cannot be generated only by the TEMPO radical, requiring the presence of NaBrO and/or NaClO in the system at pH 10 (Figure 2).
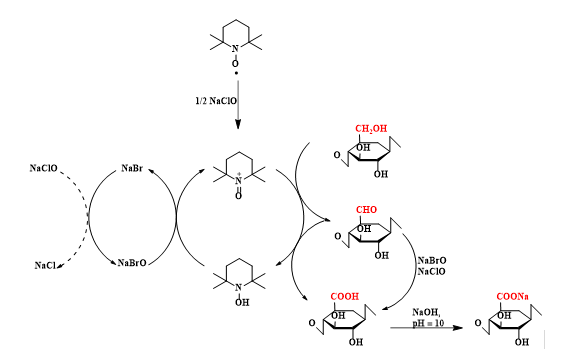
Fig. 2. Regioselective oxidation of hydroxyl groups located at C6 from the structure of cellulose to C6-carboxylic groups by means of the oxidation system TEMPO/NaBr/NaClO in water, at pH 10 - 11
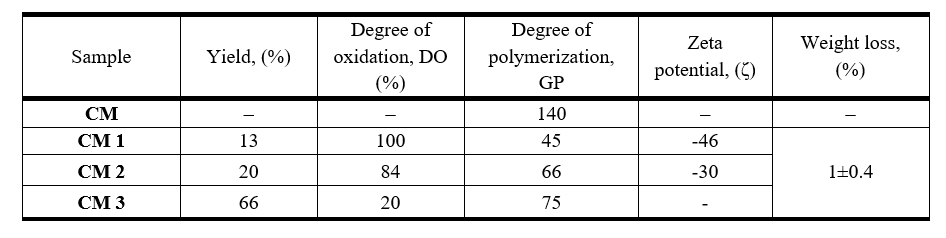
Table 1 shows yields, degrees of oxidation (OD), degrees of polymerization (GP), zeta potential, and weight loss for oxidized cellulose fractions.
Table 1. Yields, degrees of oxidation, degrees of polymerization, zeta potentials and weight loss for various samples of oxidized cellulose
The samples were characterized from several points of view:
- Nuclear magnetic resonance (13C-RMN) - figure 3
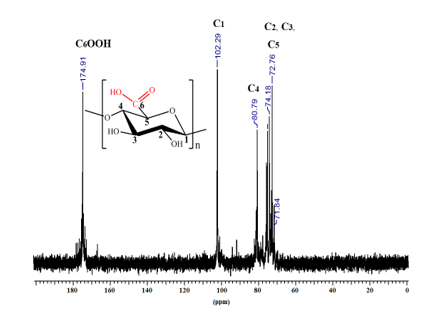
Fig. 3. 13C-NMR spectrum (in D2O) of the oxidized cellulose sample (CM 1, GO = 100%)
- FTIR spectroscopy - figure 4
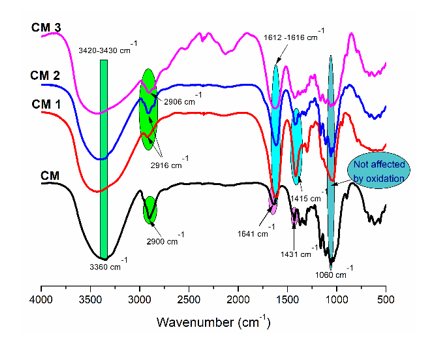
Fig. 4. FTIR spectra of non-oxidized cellulose (CM) and oxidized samples (CM 1, CM 2 and CM 3)
- Scanning electron microscopy (SEM) - figure 5
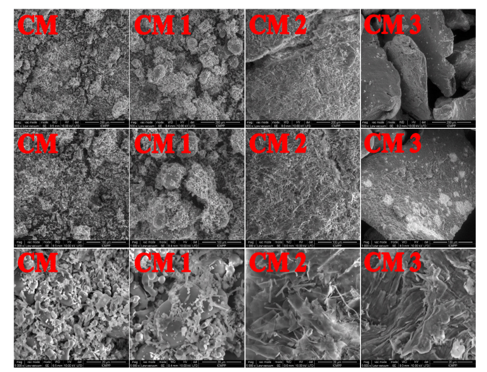
Fig. 5. SEM micrographs of microcrystalline cellulose (CM) before oxidation and its oxidized fractions (CM 1, CM 2 and CM 3), magnified 500, 1000 and 5000 times, respectively
- Thermal analysis (TGA) - figure 6
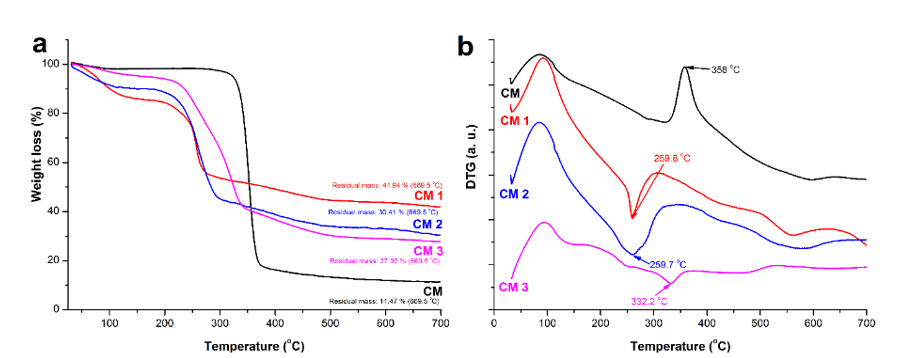
Fig. 6. Thermogravimetric curves (a) and corresponding thermogravimetric derivatives of cellulose samples before and after oxidation (b)
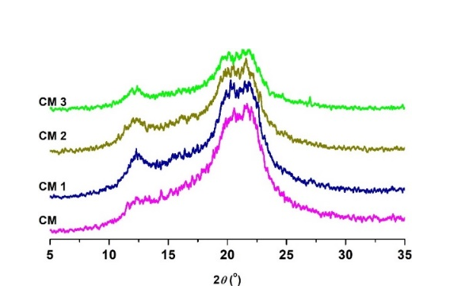
Fig. 7. X-ray diffractograms of cellulosic samples before and after oxidation
Activity 1.2. Preparation of carboxylated cellulosic substrates by functionalizing cellulosic sorts with citric acid
This activity aimed to prepare citric acid modified cellulose (CA) in order to obtain a high content of carboxylic groups on the cellulose surface. The chemical reaction that takes place on the surface of cellulose fibrils is described in Figure 8.
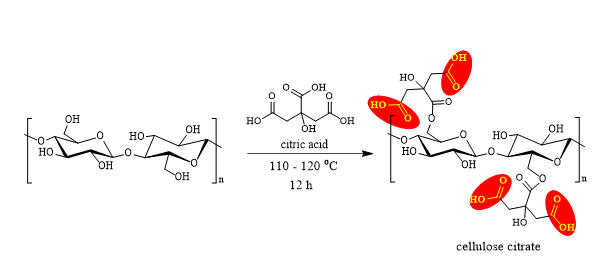
Fig. 8. Chemical reaction between cellulose and citric acid
A sample of Cotton Linters was chosen for the preparation of carboxylated cellulose substrates. Final samples were characterized from several points of view:
- FTIR spectroscopy - figure 9
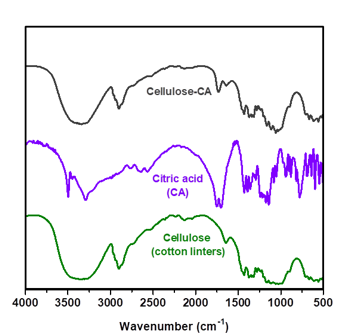
Fig. 9. FTIR spectra for cellulose sample (Cotton Linters), citric acid (CA) and cellulose modified with citric acid (Cellulose-CA)
- Dynamic light scattering method (DLS) - figure 10
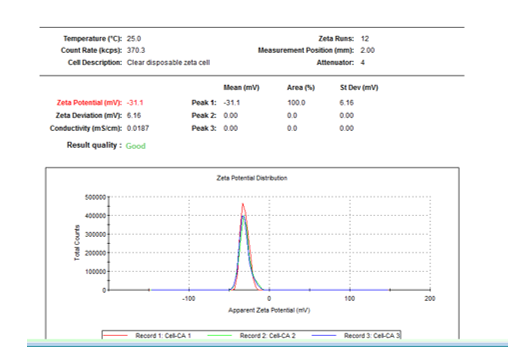
Fig. 10. Value of zeta potential for cellulose functionalized with citric acid
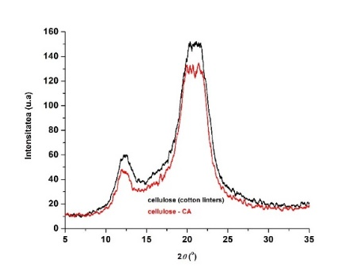
Fig. 11. X-ray diffractograms of cellulosic samples: initial cellulose and cellulose functionalized with citric acid
Activity 1.3. Preparation of nanocellulose from agricultural waste
For this research activity, we turned our attention to the preparation of nanocrystalline cellulose (CNC) starting from plant residues from hemp stems. This process involved in the first phase the separation of lignin and hemicellulose cellulose from plant residues, then the cellulose obtained was subjected to specific treatment processes in order to obtain nanocellulose. Obtaining and characterizing nanocellulose can have a significant impact on the research we undertake, being well known the benefits of using nanocellulose as an additive in composite materials, hydrogels, due to the remarkable properties that this compound transmits to final materials.
The nanocellulose extraction process consisted of several stages:
- Treatment of hemp with ethanol
- Hemp treatment with sodium hydroxide
- Treatment of hemp with sodium hypochlorite
- Treat hemp with hydrogen peroxide
Figure 12 describes in detail the transition from raw material (hemp stalks) to cellulose following chemical treatments.
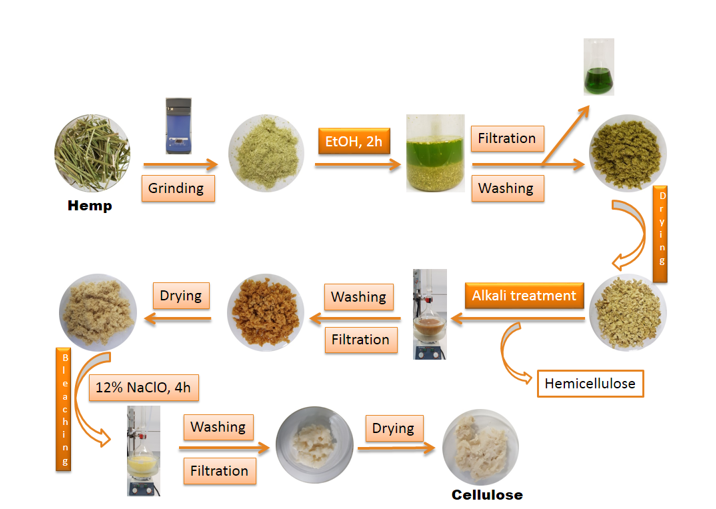
Fig. 12. Synthesis of nanocellulose from plant raw materials, from hemp stems
Oxidation of 2,2,6,6-tetramethylpiperidine-N-oxyl-mediated cellulose (TEMPO) - Figure 13 shows schematically the process of the cellulose oxidation reaction.
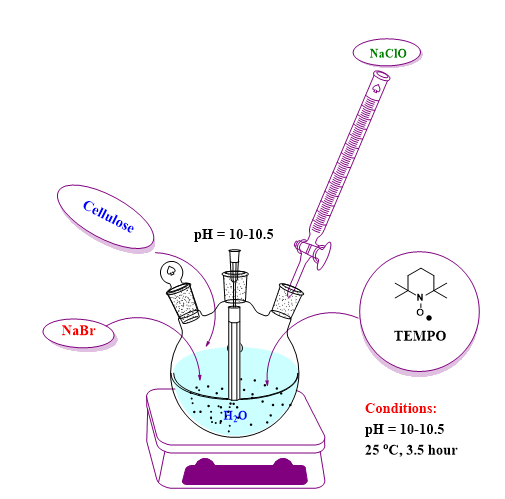
Fig. 13. Schematic representation of the working method for obtaining oxidized cellulose
The mechanism of the TEMPO radical-mediated oxidation reaction is shown in Figure 14. NaClO, used as the primary oxidant, first oxidizes the TEMPO radical to the N-oxoammonium or TEMPO+ structure, which then oxidizes the primary hydroxyl groups in cellulose to carboxylic groups, through aldehyde groups (C-6). N-hydroxy-TEMPO or its reduced form is oxidized by sodium hypobromite (NaBrO), formed in the reaction medium in the presence of sodium bromide (NaBr) and sodium hypochlorite (NaClO). Thus, TEMPO and NaBr behave as catalysts and only NaClO is consumed during oxidation. Because carboxylic groups are formed by the oxidation reaction, maintaining the reaction medium at pH = 10 is done with a small amount of 0.5 M NaOH solution. Some of the aldehyde groups are oxidized directly to carboxylic groups with NaClO and/or NaBrO.
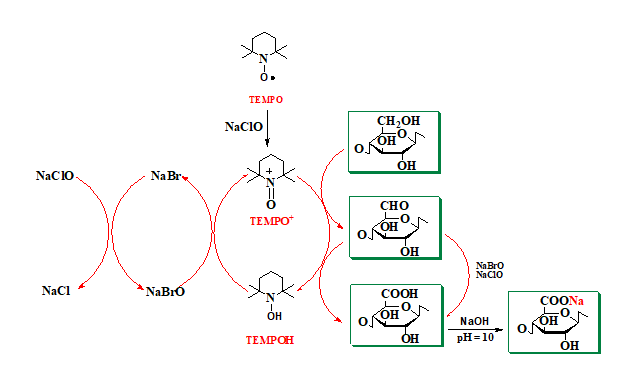
Fig. 14. Regioselective oxidation of primary alcohol groups from cellulose to carboxylic groups via the TEMPO/NaBr/NaClO oxidation system, at pH 10
The samples were characterized from several points of view:
- FTIR spectroscopy - figure 15
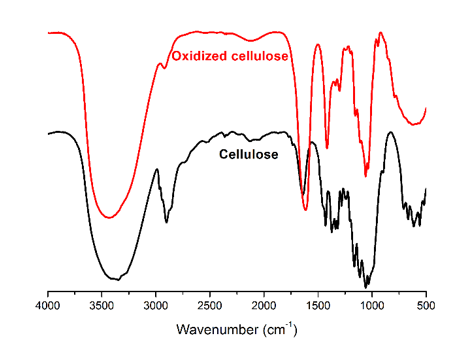
Fig. 15. FTIR spectra of cellulose and oxidized cellulose via the TEMPO/NaBr/NaClO oxidation system

Fig. 16. SEM images of hemp stems, cellulose and oxidised cellulose via the TEMPO/NaBr/NaClO oxidation system
- Transmission electron microscopy (TEM) - figure 17
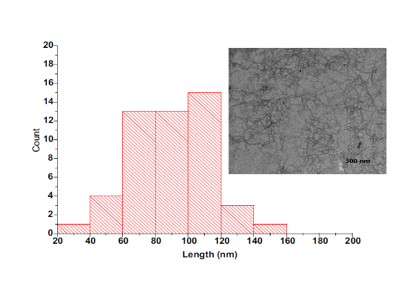
Fig. 17. Cellulose fiber length distribution and TEM image of the oxidized cellulose sample
Publications
1. S. Coseri, Insights on Cellulose Research in the Last Two Decades in Romania, Polymers, 2021, 13, 689. IF = 4.329
2. R.I. Baron, G. Biliuta,V. Socoliuc, S. Coseri, Affordable magnetic hydrogels prepared from biocompatible and biodegradable sources, Polymers, 2021, 13, 1693. IF = 4.329
3. M. Ciopec, G. Biliuta, A. Negrea, N. Duteanu, S. Coseri, P. Negrea, M. Ghangrekar, Testing of chemically activated cellulose fibers as adsorbents for treatment of arsenic contaminated water, Materials, 2021, 14, 3731. IF = 3.623
Participations at scientific events
1. G. Biliuta, R.I. Baron, S. Coseri, Advancements on the selective oxidation of cellulose, via non-persistent phthalimide N-oxyl radical, Bucharest Polymer Conference 2nd Edition, June 10 - 11, 2021, Bucharest, Romania – oral communication
2. S. Coseri, Proton conductive membranes for fuel cells: challenges when cellulose replace nafion, 13th International Conference on Physics of Advanced Materials, September 24-30, 2021, Sant Feliu de Guixols, Spain – oral communication
3. M. Asandulesa, M.E. Culica, S. Coseri, Dielectric spectroscopy investigation of novel hybrid composites obtained from cellulose derivatives doped with 1-hydroxybenzotriazole, 13th International Conference on Physics of Advanced Materials, September 24-30, 2021, Sant Feliu de Guixols, Spain – oral communication
4. G. Biliuta, R.I. Baron, S. Coseri, Cellulose-carbon nanotubes hybrid materials acting as electromagnetic shielding materials, Advanced Materials in Modern Medicine – International Congress ”By promoting excellence we prepare the future”, March 1–3, 2021, Iasi, Romania - poster
5. R.I. Baron, M.E. Culica, G. Biliuta, S. Coseri, Hybrid cellulose - carbon nanotubes composites with electromagnetic shielding properties, The 1st National Conference with international participation, One Health Approach in a Changing World, November 4-5, 2021, Chisinau, Republic of Moldova – poster
6. M.E. Culica, G. Biliuta, S. Coseri, Cellulose functionalization for technical applications: fuel cell membranes, The 1st National Conference with international participation, One Health Approach in a Changing World, November 4-5, 2021, Chisinau, Republic of Moldova – poster
7. G. Biliuta, S. Coseri, New pathways in cellulose-derivative production with applications for hybrid materials, The 1st National Conference with international participation, One Health Approach in a Changing World, November 4-5, 2021, Chisinau, Republic of Moldova – poster | 





