O1. Nanostructured cellulose interface preparation, the base of sensor (2020-2021)
This objective is considered the first step of the overall biointerface process. Several types of cellulosic samples, including cotton linters, microcrystalline, never-dried sulfite pulp, and viscose, were tested as potential sources to prepare cellulose-based derivatives, able to be used in homogeneous systems.
The chosen derivatization procedure implies the chemical oxidation using 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) radical, sodium hypochlorite, and sodium bromide. Following this treatment, each cellulose sample was processed in such a way as to maximize the content of water-soluble fractions. Therefore, for each cellulose type (except viscose), we could isolate three oxidized samples, see Fig. 1.
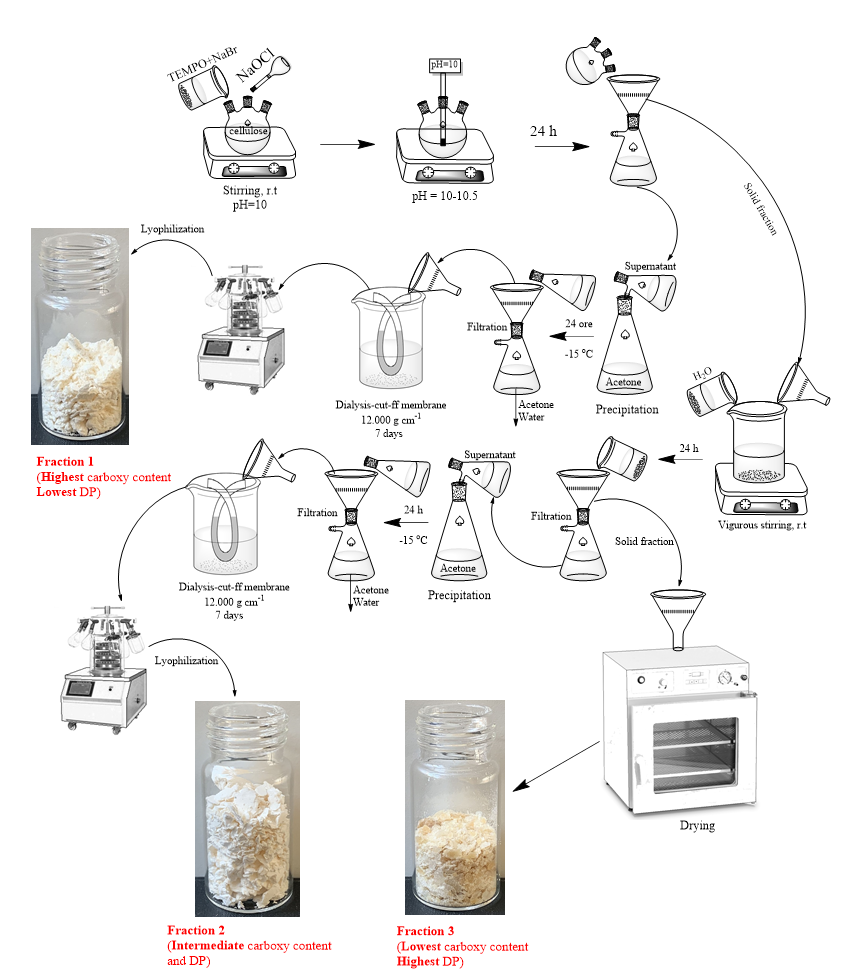
Fig. 1. General scheme for the production cycle of oxidized cellulose fractions with different water solubility.
Furthermore, Fig. 2 shows the appearance of 1% suspensions of CL, CL 1, CL2 and CL 3 samples, in several solvents, like: water, DMF, acetone, ethanol, and DMSO.
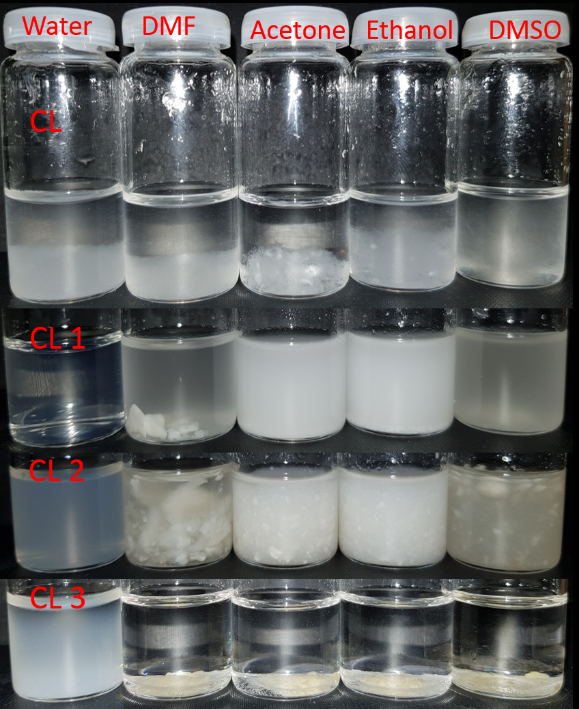
Fig. 2. Photographs of 1% suspension stability of CL and the isolated oxidized fractions (CL 1, CL 2, CL 3) in water, DMF, acetone, ethanol and DMSO.
It can be observed, that oxidized fractions reach in carboxyl groups, CL 1 and CL 2 displays a good solubility in water; The other oxidized fraction, CL 3, with less carboxyl groups incorporated, do not dissolve into water, forming only a suspension. CL 1 sample exhibit a good dispersibility also in acetone, ethanol and DMSO, but not in DMF.
The solubility limit in water of the two oxidized fractions was additionally checked by using low-voltage electron microscopy (LVEM). LVEM is a powerful transmission electron microscope able to acquire high-resolution images of biological and organic samples, combining the capabilities of the TEM, SEM and STEM techniques. As the Fig. 3 reveals, the solubility of the CL 1 sample in water is excellent until 0.8% concentration, while the sample CL 2 displays a good water solubility in the range of concentrations until 0.2%. At higher concentrations, above the mentioned values, both samples tend to form agglomerations, or clusters visible under a microscope, indicating that the solubility limit has been reached.
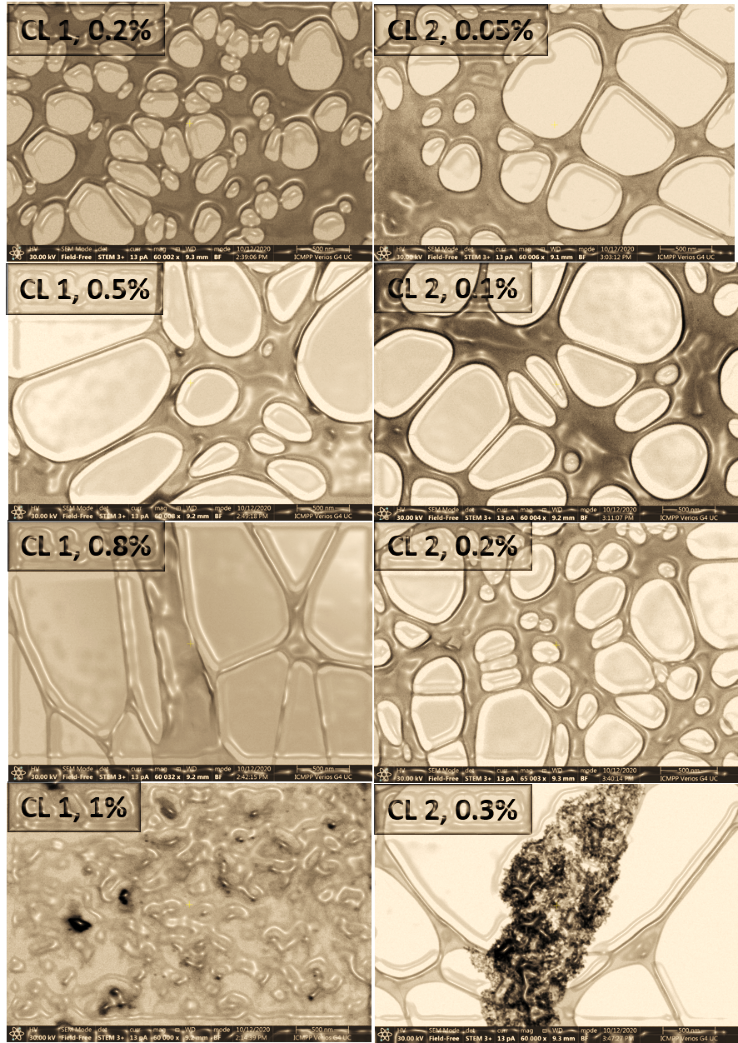
Fig. 3. STEM images of aqueous solutions of different concentrations of CL 1 and CL 2 samples.
O2. Linking the reactive sites on the sensor base surface (2021)
This step imply the use of the previously synthetized surfaces as platforms for the anchoring of binding sites for further immobilization of hIgG.
To achieve the base surface, the COOH groups previously introduced in the anhydroglucose unit of cellulose were first activated. The activation is performed by using a classical EDC/NHS coupling protocol, as depicted in the Fig. 4.
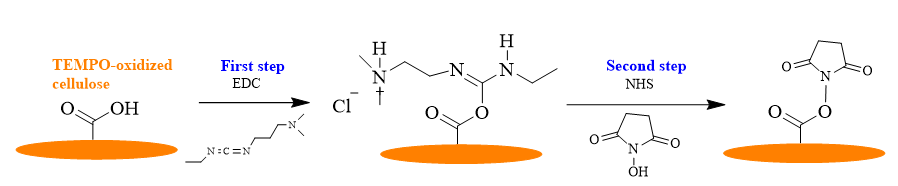
Fig. 4. Schematic representation of COOH groups activation from TEMPO-oxidized cellulose by using the EDC/NHS protocol.
The second step consist on the hexamer short peptide HWRGWV attachement onto the activated cellulose surface. The process has been monitored inside the QCM-D instrument, the resulted films being analysed by means of atomic force microscopy (AFM), Fig. 5.
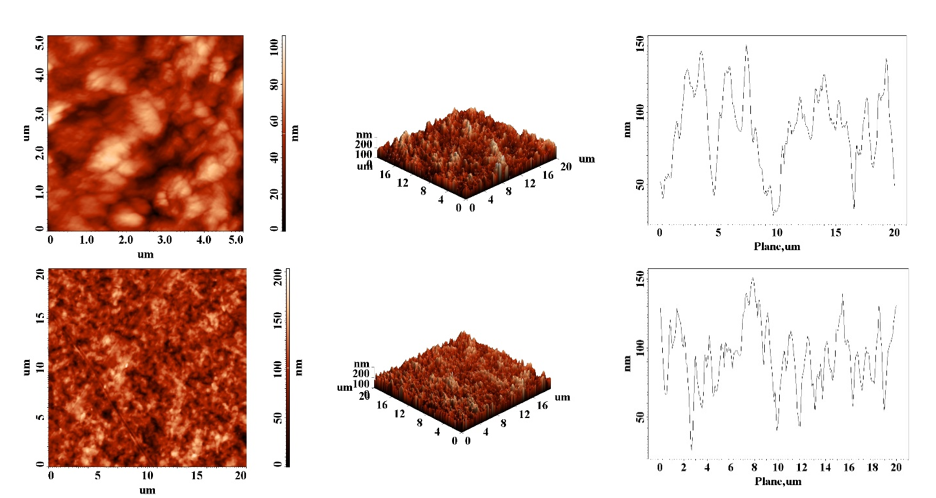
Fig. 5. Cellulose films morphology before (up) and after (down) TEMPO-oxidation, EDC/NHS activation and coupling with peptide hexamer HWRGWV.
Publications
1. R.I. Baron, S. Coseri, Preparation of water-soluble cellulose derivatives using TEMPO radical-mediated oxidation at extended reaction time, Reactive and Functional Polymers, 157, 104768, 2020. IF = 3.975
2. M.E. Culica, A.L. Chibac-Scutaru, T. Mohan, S. Coseri, Cellulose-based biogenic supports, remarkably friendly biomaterials for proteins and biomolecules, Biosensors and Bioelectronics, 182, 113170, 2021. IF = 10.618
3. I.A. Duceac, L. Vereștiuc, A. Coroaba, D. Arotăriței, S. Coseri, All-polysaccharide hydrogels for drug delivery applications: Tunable chitosan beads surfaces via physical or chemical interactions, using oxidized pullulan, International Journal of Biological Macromolecules, Volume 181, 1047-1062, 2021. IF = 6.953
4. O. Burduniuc, A.C. Bostanaru, M. Mares, G. Biliuta, S. Coseri, Synthesis, characterization, and antifungal activity of silver nanoparticles embedded in pullulan matrices, Materials, 14, 7041, 2021. IF = 3.623
Participations at scientific events
1. R.I. Baron, G. Biliuta, S. Coseri, Preparation of water-soluble carboxylated cellulose derivatives as efficient hydrogel promoters, Bucharest Polymer Conference 2nd Edition, University POLITEHNICA of Bucharest, Romania, June 10-11, 2021 - oral communication
2. I.A. Duceac, S. Coseri, Oxidized pullulan for fine-tuning a chitosan-based drug delivery system, Bucharest Polymer Conference 2nd Edition, University POLITEHNICA of Bucharest, Romania, June 10-11, 2021 - oral communication
3. A.L. Chibac-Scutaru, V. Melinte, S. Coseri, Cellulose-derived platforms for targeted technological applications, 13th International Conference on Physics of Advanced Materials, Sant Feliu de Guixols, Spain, September 24-30, 2021 - oral communication
4. R.I. Baron, G. Biliuta, S. Coseri, Hydrogels based on PVA and pullulan with self-healing behavior, Advanced Materials in Modern Medicine – International Congress ”By promoting excellence we prepare the future” Iasi, Romania, March 1–3, 2021 - poster
O3. Sensor testing for specific detection of proteins (2022)
The oxidation of cellulose with sodium periodate usually undergoes in water. Due to the low reactivity of cellulose, this reaction implies considerable amounts of periodate, compared to the mass of cellulose; moreover, the oxidation times used are of the order of hours or even days. The large amount of periodate consequently induces a decrease of the oxidation efficiency, as well as the generation of significantly large amounts of chemical waste containing iodine.
Proteins can be immobilized on cellulosic surfaces that contain aldehyde groups, through covalent bonds that appear by interacting with the amino groups that are abundantly present in the protein structures. There are very few scientific works in this field, which makes us believe that our work can open a vast field of research in the area of the immobilization of different proteins on cellulosic surfaces previously functionalized by the introduction of aldehyde groups.
The initial stage consisted in the selective oxidation of cellulose in the presence of sodium periodate is illustrated in Figure 1.
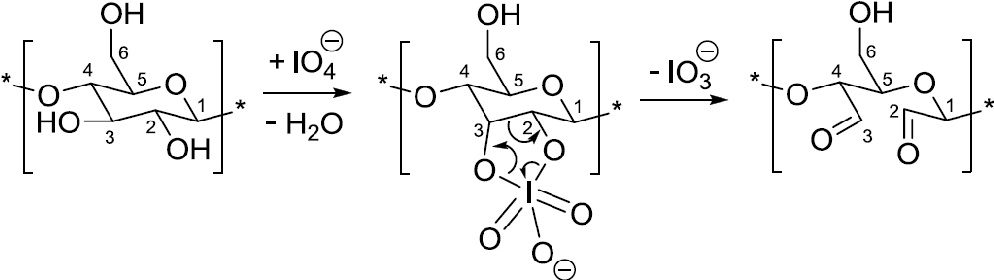
Fig. 1. Schematic illustration on the synthesis of dialdehyde cellulose (DAC).
The presence of the aldehyde groups introduced after the reaction was highlighted by FTIR spectroscopy. The spectrum of the reaction product clearly shows a new peak at 1721 cm-1 characteristic for the aldehyde groups, Figure 2.
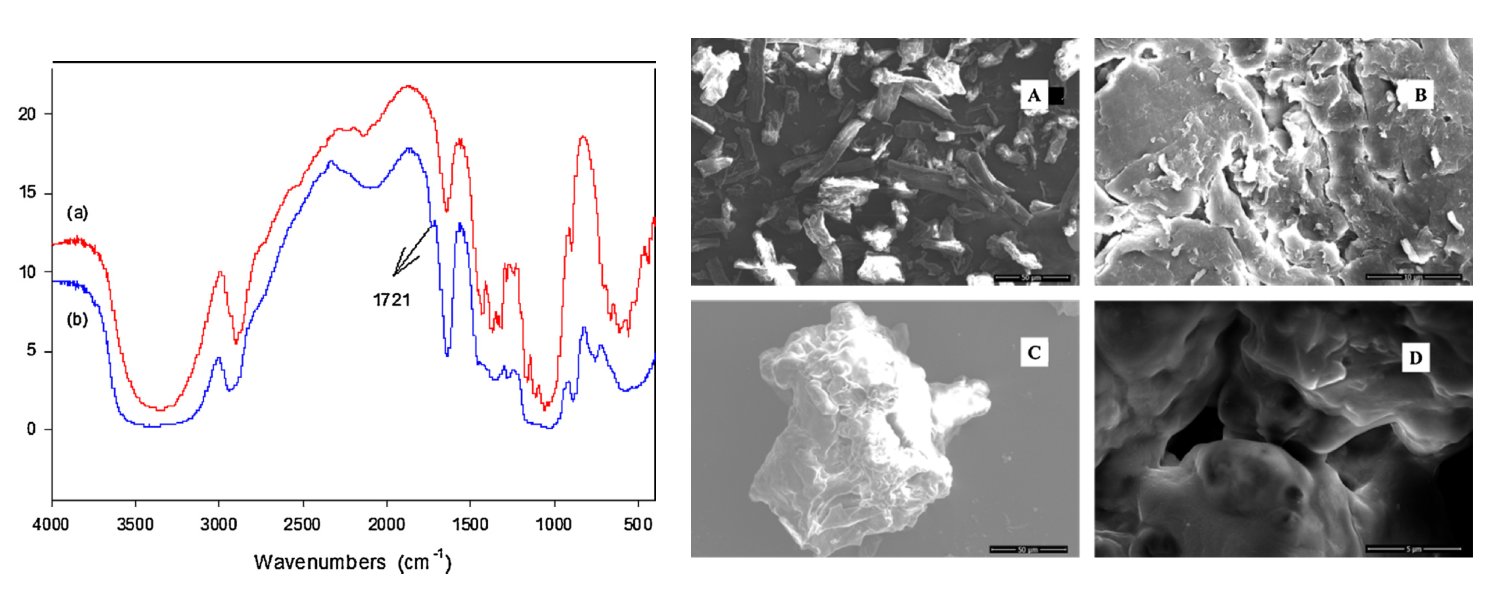
Fig. 2. FTIR spectra for raw cellulose (a) and dialdehyde cellulose DAC (b) and the SEM images recorded for cellulose (A), activated cellulose with NaOH (B), and DAC (C and D).
A new protocol for protein immobilization on biopolymer-based platforms
The strategy for protein immobilization on substrates based on natural resources developed within this project can be described in three stages, as follows:
- Preparation of the cellulosic "foundation", by carrying out oxidation reactions and introducing carboxylic and/or anhydride groups
- Preparation of the "sites" of interest for protein immobilization, according to the "key-and-lock" principle
- Effective immobilization of the proteins (immunoglobulin)
These stages presented above are represented synthetically in Figure 3.
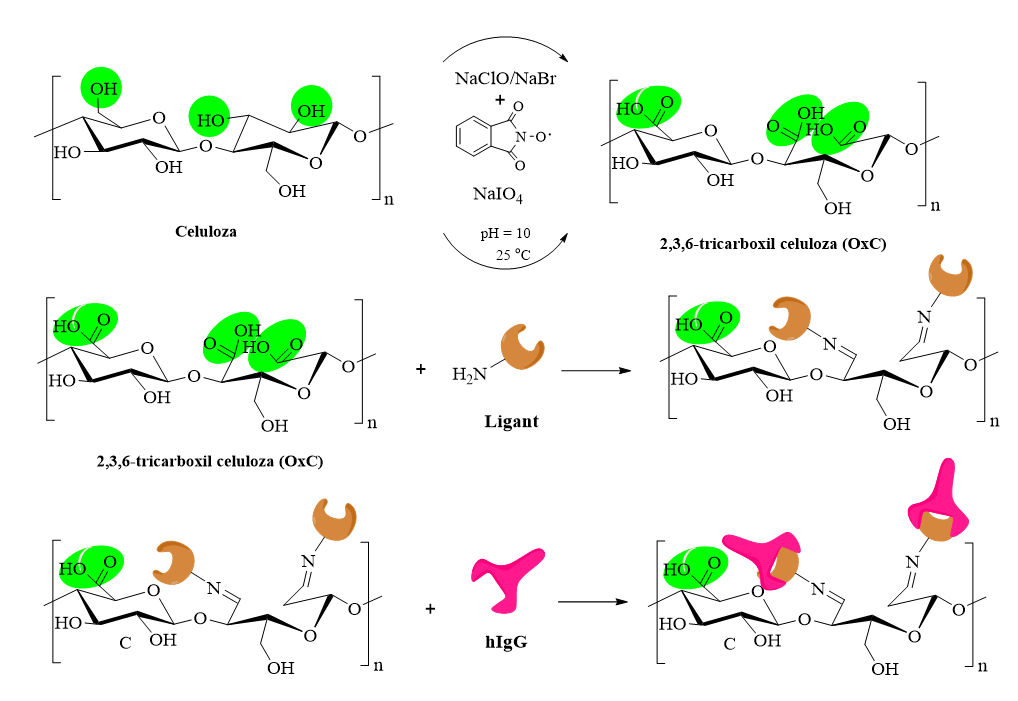
Fig. 3. Schematic representation of the protein immobilization protocol using natural platforms.
BSA and IgG adsorption, followed by SPR
The SPR technique was used to measure the BSA adsorption at pH 4 and 5, when a 10 mM sodium acetate buffer was used, and pH 6.2 and 7.4, in which case a phosphate buffer we used. The ionic strength of the buffer solutions was adjusted by adding 50 mM sodium chloride solutions. A BSA solution (0.1 mg/mL) was used to perform adsorption experiments on cellulose films obtained from i) TMSC, and ii) oxidized films using the NHPI/NaClO/NaBr process. The adsorption process was monitored until the plateau was reached, then the corresponding buffer solution was introduced along the route, with the role of washing the possible surplus of unbound (unabsorbed) BSA. In a similar way, we performed the IgG adsorption experiments, using the cellulosic substrates. The main results are presented in Figure 4.
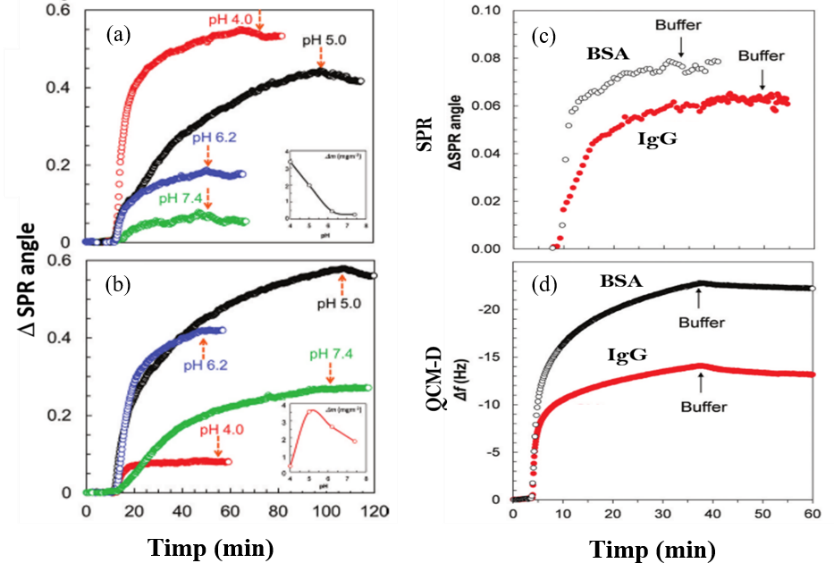
Fig. 4. The SPR sensograms recorded for the adsorption of BSA (a) and IgG (b) on the oxidized cellulose films, at different pH values, respectively, and the SPR sensograms (c) and QCM-D curves (d) for the adsorption of BSA and IgG on the oxidized cellulose films.
As can be seen, BSA adsorption on the oxidized cellulosic substrate, using the protocol described in the previous sections, i.e., NHPI/NaClO/NaBr, has high values at pH=4, due to the presence of negative COO- groups, capable (at this pH) of binding the positive groups from BSA. On the other hand, at pH=7.4, the adsorbed mass is much lower. IgG adsorption is the highest at ph=5, and at pH=6.2, close to the isoelectric point of IgG, while at extreme pHs of the isoelectric point, i.e., 4 and 7.4, the adsorption is reduced.
Published papers:
1. X. Yao, S. Zhang, L. Qian, N. Wei, V. Nica, S. Coseri, F. Han, Super stretchable, self-healing, adhesive ionic conductive hydrogels based on tailor-made ionic liquid for high-performance strain sensors, Advanced Functional Materials, 32, 2204565, 2022. IF = 19.924

2. I.A. Duceac, F. Tanasa, S. Coseri, Selective oxidation of cellulose - A multitask platform with significant environmental impact, Materials, 15, 5076, 2022. IF = 3.748
3. G. Biliuta, A.-C. Bostănaru-Iliescu M. Mareș, C. Pavlov-Enescu, V. Năstasă, O. Burduniuc, S. Coseri, Antibacterial and antifungal silver nanoparticles with tunable size embedded in various cllulose-based matrices, Molecules, 27(19), 6680, 2022. IF = 4.927
Papers under review:
1. N. Li, S. Zhang, Y. Liu, V. Nica, S. Coseri, Cellulose amidation modification via oleic acid for hydrophobicity improvement, Carbohydrate Polymers, 2022. IF = 11.072
2. S. Cui, S. Zhang, S. Coseri, An injectable and self-healing cellulose nanofiber-reinforced alginate hydrogel for bone repair, Carbohydrate Polymers, 2022. IF = 11.072
3. I.A. Duceac, S. Coseri, Biopolymers and their derivatives: key components of advanced biomedical technologies, Biotechnology Advances, 2022. IF = 17.681
Participations at scientific events:
1. I.-S. Trifan, V. Melinte, A.L. Chibac-Scutaru, S. Coseri, Effect of functionalization degree of celulose derivatives on their properties and photopolymerization profile, A XXXVI-a Conferinta Nationala de Chimie, Calimanesti-Caciulata, Valcea, Romania, October 4-7, 2022
2. I.A. Duceac, S. Coseri, Chitosan-based hydrogels: from materials science to drug delivery systems, A XXXVI National Chemistry Conference, Calimanesti-Caciulata, Valcea, Romania, October 4-7, 2022,
3. I. A. Duceac, S. Coseri, Fine-tuning the hydrogels porosity: a reliable strategy for controlling drug delivery behavior, 32nd Annual Conference of the European Society for Biomaterials - ESB 2022, Bordeaux, France, September 4-8, 2022.
4. M. E. Culica, A. L. Scutaru, M. Asandulesa, S. Coseri, Cellulose-derived platforms for emergent energetic applications, Polymer Network Group-PNG2022, Rome, Italy, June 12-16, 2022.
5. R.I. Baron, G. Biliuta, S. Coseri, Expanding the cellulose versatility towards new selfhealable hydrogels fabrication, Congresul international pregatim viitorul promovand excelenta, Editia a XXXII-a, Iasi, Romania, February 28 – March 2, 2022.
"As a young researcher, former PhD student, I had the chance to be a member of the HYSENSE - PED project and I would like to acknowledge the support of the project director, Dr. Sergiu Coseri, the UEFISCDI, and the Romanian Ministry of Research and Innovation. As a member of this project, I had the unique opportunity to attend the 32nd Annual Conference of the European Society for Biomaterials. This is a very prestigious conference, one of the top scientific events in the region and the world map in the field of advanced materials for biomedical applications (tissue engineering, biomedical sensors and soft electronics, advanced characterization techniques, 3D printing and bioinks, controlled/targeted drug and gene delivery systems, cell encapsulation and organoids, implants, medical devices etc.). This gave me the chance to both share my research (poster presentation on the study Fine-tuning the hydrogels porosity: a reliable strategy for controlling drug delivery behavior) and meet over 1000 participants (oral and poster presentations) from around the world. Advanced materials and innovative solutions were discussed and debated during four days of the conference, aiming to solve some of the challenges in this field: biocompatibility issues, structure-properties correlations, clinical difficulties and regulations, translational opportunities and barriers etc. The honourable plenary speakers (Pr. Molly Stevens, Professor Matthias Lutolf, Professor Kristi Anseth, Professor José-Alain Sahel, Professor Marja A. Boermeester) and ESB awardees (Pr. Abhay Pandit, Pr. Joachim Kohn, Dr. Khoon Lim, Pr. Rui Reis) shared not only next-level scientific knowledge, but also examples of developing lab infrastructure, transitioning exceptional ideas towards entrepreneurship, and forming long-term relationships for faster and smoother professional and scientific advancement" - I.A. Duceac, member of HYSENSE project
|






