
|


|
|||||
| Home | |||||
| Team members | |||||
| Project abstract | |||||
| Objectives | |||||
| Dissemination | |||||
|
|||||
| Contact |
Stage 2021: ![]()
Objectives:
Stage 2/2021: The obtaining and characterization of the semi–interpenetrating polymer networks (S–IPNs). The thermal and fire behavior of the S–IPNs
Activity 2.1. The obtaining and characterization of the semi–interpenetrating polymer networks (S–IPNs) based on aromatic oligophosphonate (OP) and an epoxy resin
Activity 2.2.The microbiological testing of the S–IPNs
Activity 2.3. The thermal and fire behavior of the S–IPNs
Scientific Report - Stage 2021: ![]()
Stage 2021 Summary
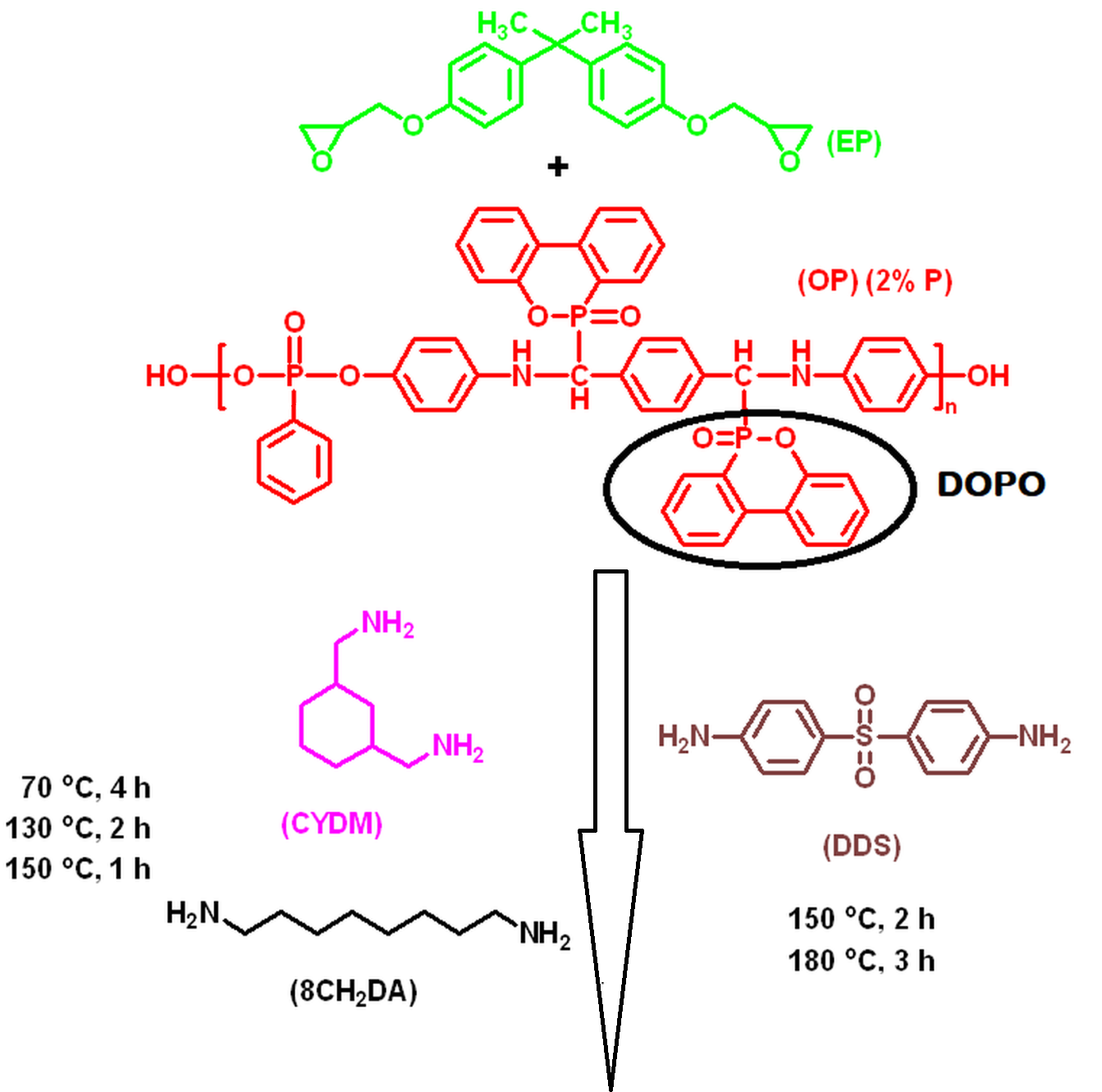
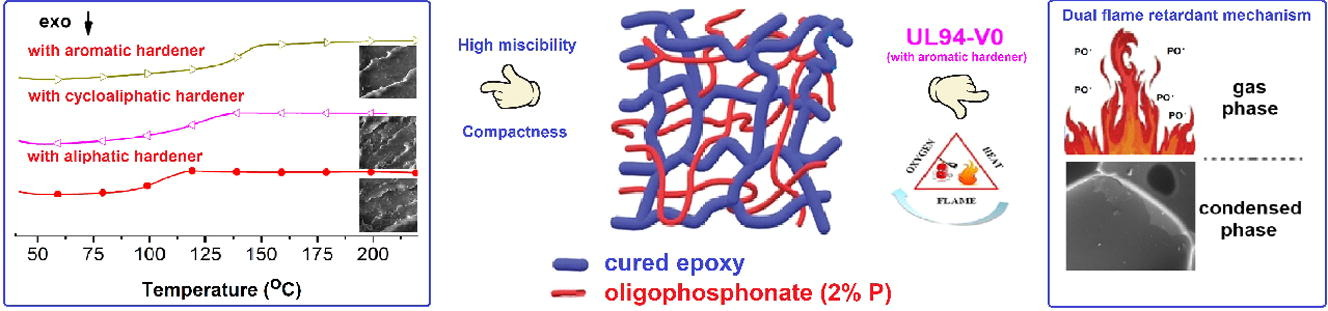
The semi-intepentrating polymer networks (S–IPNs) were obtained by mixing the epoxy resin, diglycidyl ether of bisphenol A (EP) with different amounts of OP under heating and stirring, followed by curing with: 4,4’–diaminodiphenylsulfone (DDS), 1,3–bis(aminomethyl)cyclohexane (CYDM) and octamethylenediamine (8CH2DA). The quantity of OP was calculated in order to obtain final products with 2 wt% phosphorus. The S-IPNs were characterized by FTIR, DSC and SEM-EDX methods. The S–IPNs DSC curves showed a good miscibility of the OP in the cured epoxy resin..
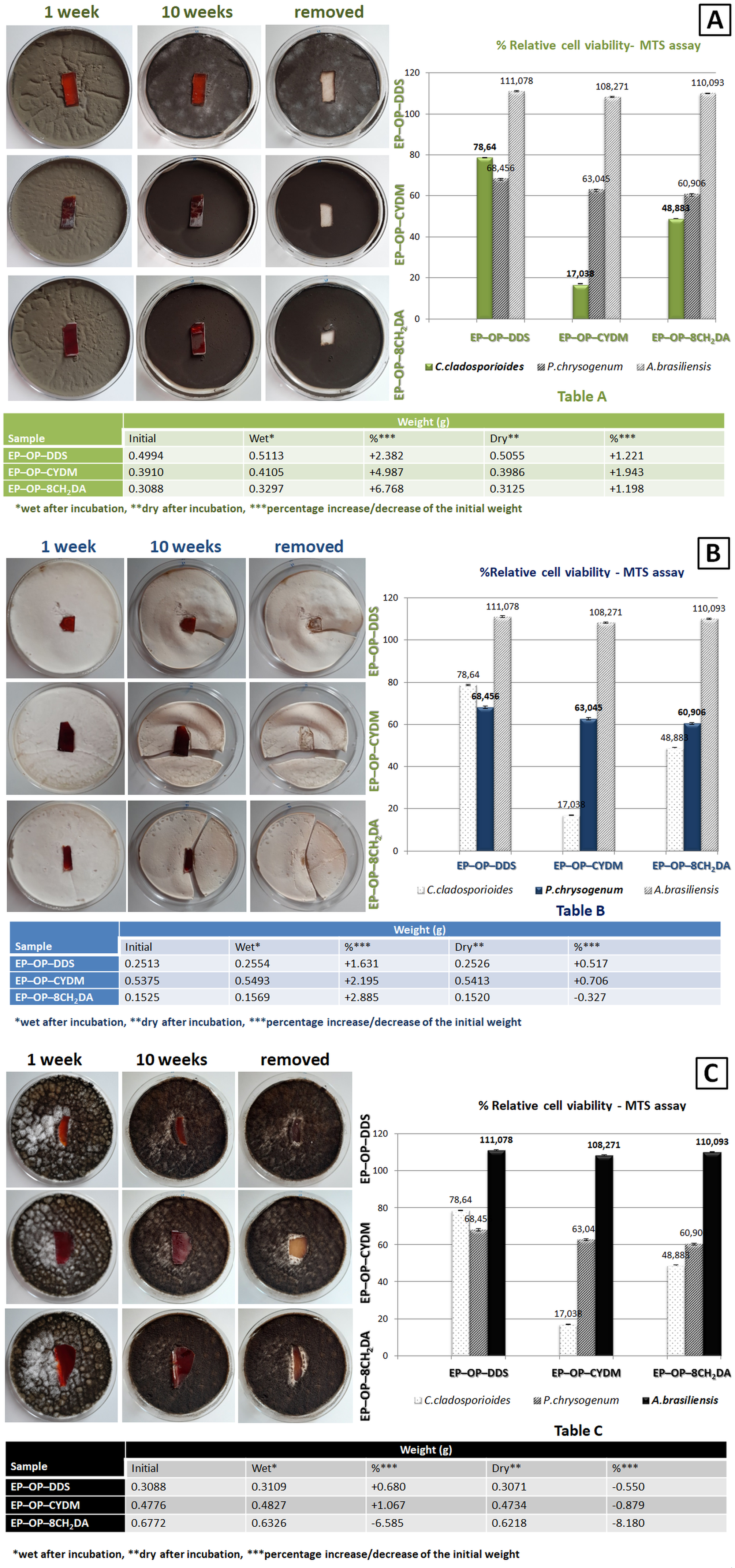
Since the S–IPNs are intended as future wood protective coatings, the samples microbiological resistance was tested against three wood decaying fungal strains: Penicillium chrysogenum, Cladosporium cladosporioides and Aspergillus brasiliensis. During the decay test (10 weeks) all surfaces of samples were not covered by the fungal colonies, except samples exposed to A. brasiliensis where the edges where slightly covered.
The sample EP–OP–CYDM reduced the population of P. chrysogenum at 17 %. In the case of the samples incubated with A. brasiliensis, there was not noticed a fungistatic activity. Also it was noticed that the S–IPNs had a greater fungistatic activity than their cured counterparts without OP, due to the presence of phosphorus. The samples presented instead fungicidal properties when incubated with C. cladosporioides and A. brasiliensis and partially for P. chrysogenum, the areas under the samples remaining clear of fungal colonies after 10 weeks of incubation where the samples adhered perfectly to the culture media.
In order to study the thermal behavior of the S-IPNs, TGA measurements were assessed in both inert and thermo–oxidative atmospheres. The non–isothermal kinetic parameters of the thermal degradation reactions were calculated with the isoconversional methods of Friedman and Ozawa–Flynn–Wall and indicated that the structures decompose following complex mechanisms in two or three stages depending on the chemical structure of the curing agent. SEM, TGA, DSC and MCC methods demonstrated that S–IPNs are highly compact networks. The flame retardant capacity of the structures was assessed with the UL 94–VB burning test and MCC method. TGA–FTIR and Py–GC–MS analyses were used to determine the evolved gaseous fragments of the samples during thermal degradation. A thermal degradation mechanism was proposed.
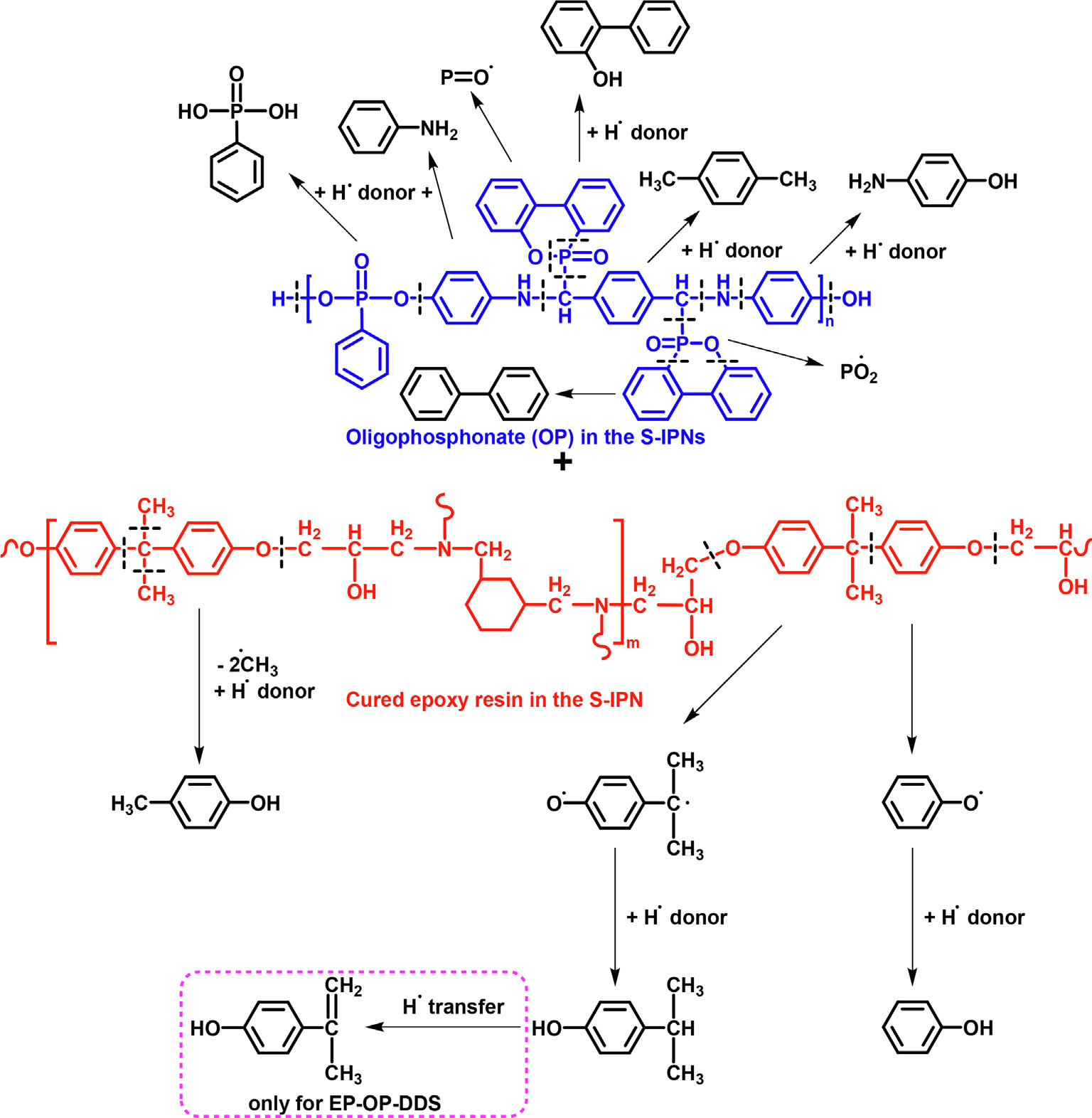
Through the MCC method it was shown that the S-IPNs possess good flame retardant capacity, as compared to the OP free cured matrices. Only structure EP-OP-DDS passed the UL 94-VB burning test with a V-0 rating. All obtained data indicated that the EP–OP–DDS system may be considered the best candidate in different applications. For example it could be used as/or in a flame retardant coating, due to the phosphine oxide group generating miscibility and adhesion to various substrates, or as a matrix for flame retardant composites. The obtained data also demonstrated that the flame retardant mechanism of the S–IPNs occurred in both gas and condensed phase.
| Designed & Maintained by CLICK NET SOLUTIONS |




