
|


|
|||||
| Home | |||||
| Team members | |||||
| Project abstract | |||||
| Objectives | |||||
| Dissemination | |||||
|
|||||
| Contact |
Stage 2020: ![]()
Stage 1/2020: Obtaining of the semi-interpenetrated polymer networks (S-IPN) based on crosslinked epoxy resin and oligophosphonate and characterization of the obtained materials
Activity 1.1.Scientific documentation:
- Creation of a database with recent information in the field of oligophosphate monomers and polymers, as well as epoxy resins (books, reviews and scientific articles, patents);
- Analysis of informational data
Activity 1.2. Investigation of modern instrumental techniques and methods:
- Selection based on literature information of investigation and characterization methods
Activity 1.3. Synthesis and structural characterization of the precursor to the oligophosphonate; Synthesis and structural characterization of the synthesized oligophosphonate:
- Purification of monomers and solvents in order to prepare the precursor synthesis;
- Monomers will be synthesized by classical methods of polycondensation and addition
Scientific Report - Stage 2020: ![]()
Stage 2020 Summary
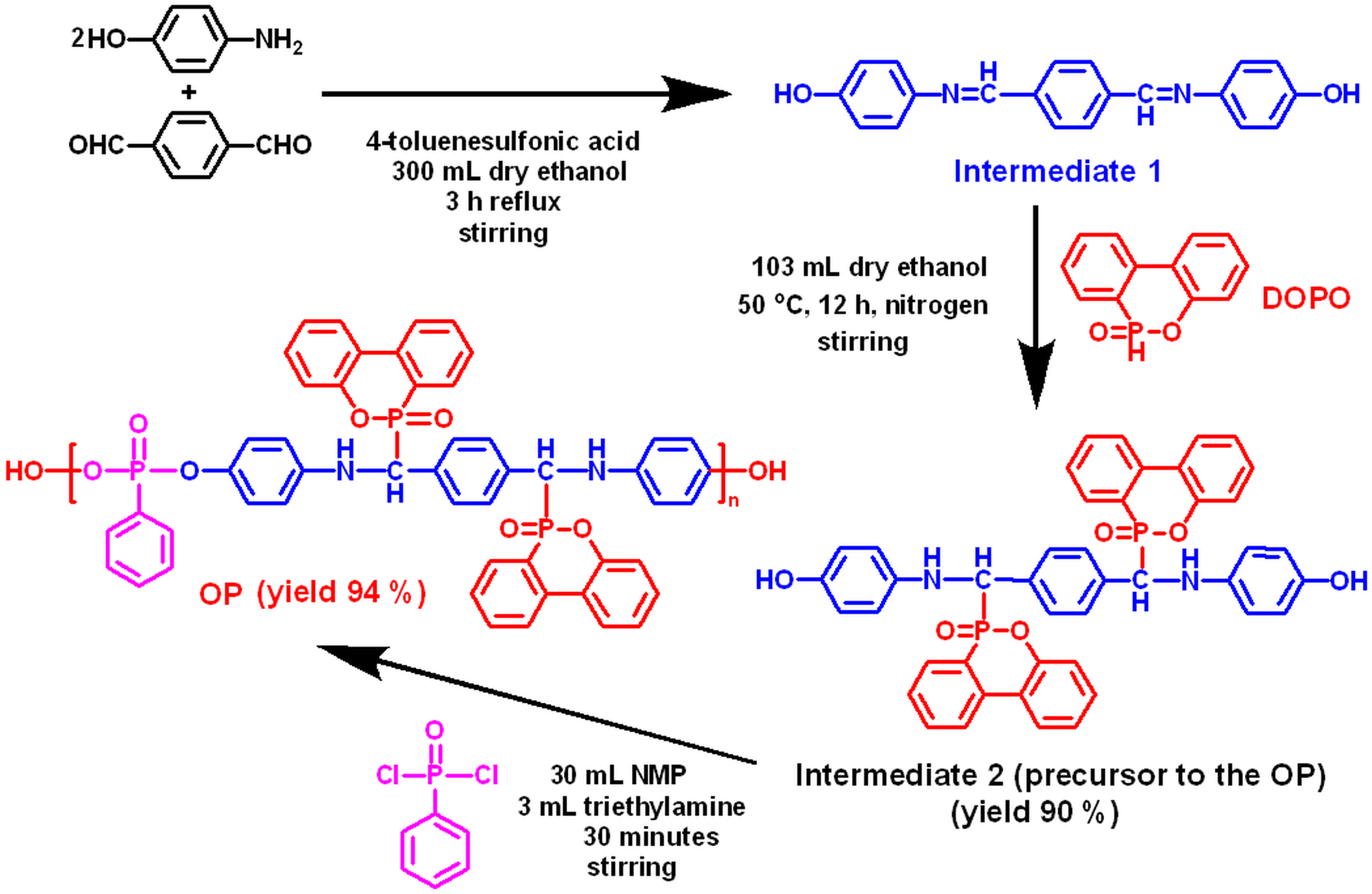
4,4’-Terephthalylidene–bis(p-hydroxyaniline) (Intermediate 1), was synthesized by reacting 4–aminophenol (0.2 mol), terephthalaldehyde (0.1 mol) and a catalytic amount of 4-toluenesulfonic acid dissolved in dry ethanol.
The synthesis of the precursor to the oligophosphonate (yield 90%) was achieved by reacting Intermediate 1 with DOPO. Characterization: FTIR (KBr pill, cm–1): 3265 (NH), 3060 (C-H aromatic), 1477 (P–Ar), 1218 and 1142 (P=O), 1043 (P–O–C), 914 (P–O-Ar), 753. 1H-NMR (400 MHz, DMSO-d6, d, ppm): 8.50 (m, 2H), 8.17 (m, 4H), 7.88 (m, 2H), 7.68 (m, 2H), 7.56 (m, 2H), 7.42 (m, 2H), 7.34 (m, 4H), 7.18 (m, 2H), 6.54 (m, 8H), 6.1 and 5.6 (m, 2H, N–H), 5.4 and 4.9 (m, 2H, CH–P).
The oligophosphonate (OP) was synthesized by solution polycondensation of equimolar amount of the precursor with phenylphosphonic dichloride and was characterized by 1H-NMR, 31P-NMR, FTIR, SEM-EDX and GPC methods.
| Designed & Maintained by CLICK NET SOLUTIONS |




