About the project
Tubulin, the repeating subunit of microtubules (MTs), and R2 ribonucleotide reductase (RNR) are validated molecular targets for anticancer drugs, as they play crucial roles in cell proliferation and other vital cellular processes.
MT-targeted agents (MTAs) and R2 RNR inhibitors lead to disruption of cell cycle progression and cell death via cell cycle arrest in G2/M and S-phase, respectively. We found recently that copper(II) compexes with indolobenzazocine-based Schiff bases show good antiproliferative activity and act as colchicine site inhibitors, while highly antiproliferative copper(II) complexes with thiosemicarbazones (TSCs), sharing with the previous agents a similar tridentate binding motif, had both tubulin- and R2 RNR inhibitory activity. These latter compounds impaired different phases of the cell cycle and were without precedence in the literature.
The aim of Metubin project is to further refine the electronic and geometric structures of the two prototypes as inhibitors of tubulin polymerization or as dual acting agents and increase their antiproliferative activity. The structural modification of the two prototype molecules will be performed via four sequential iterations by attachment of electron withdrawing and electron donating groups as well as solubilizing substituents, following initial in silico screening. In addition, by using the potential of coordination chemistry new ways to create globular shapes which will better fit into the 3D colchicine pocket will be exploited, followed by full analytical, spectroscopic and structural characterization and biological evaluation. The lead drug candidate will be tested in vivo.
These ambitious objectives will be achieved by a competent, well-structured team from Petru Poni Institute of Macromolecular Chemistry, with top chemistry expertise and infrastructure, under the guidance of a recognized specialist in medicinal chemistry from the University of Vienna, and with strong external collaborative support.
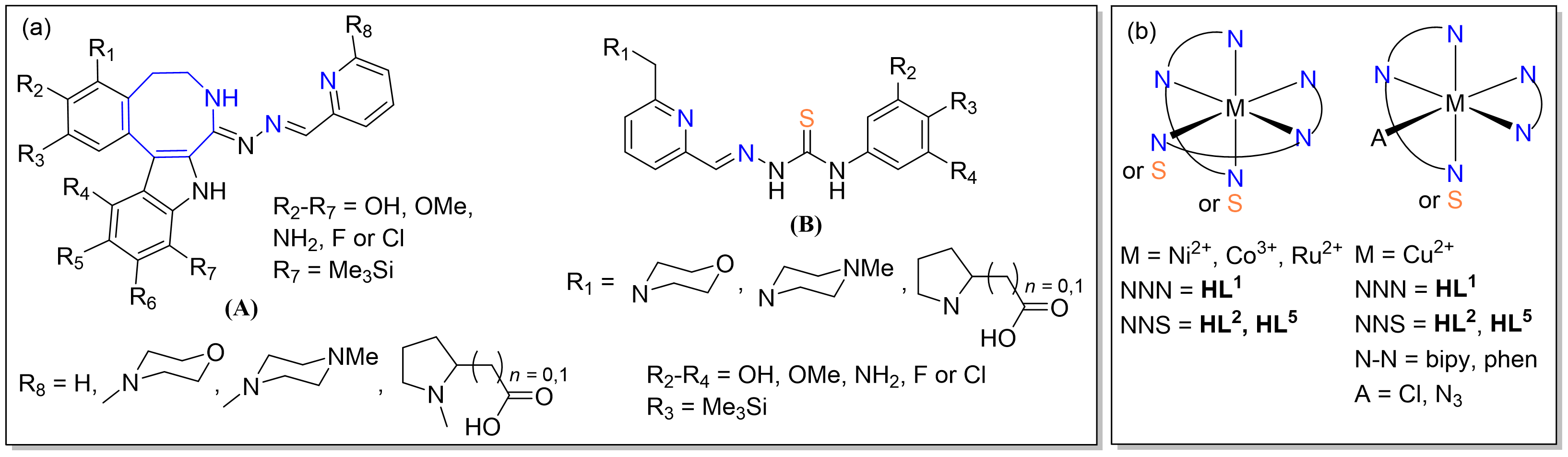
Envisaged structural modification of the two main prototypes based on 5,6,7,9-tetrahydro-8H-indolo[3,2-e]benzazocin-8-one (A) and TSCs (B) (a) and metal complexes thereof (b)
|






