
|
| Home | |||||
| Team Members | |||||
| Project Abstract | |||||
| Objectives | |||||
| Dissemination | |||||
|
|||||
| Contact |
Project Abstract: ![]()
The present project fully addresses the main objectives of Environment & Climate Action area of HORIZON 2020, which aims at achieving of resource, water efficient and climate change resilient economy and society, protecting and sustainable managing natural resources and ecosystems and ensuring a sustainable supply and use of raw materials, in order to meet the needs of a growing global population within the sustainable limits of the planet's natural resources and eco-systems. It is expected that climate-related expenditure should exceed 35% of the overall Horizon 2020 budget, including mutually compatible measures improving resource efficiency.
The world human population has substantially increased since the 15th century and, consequently, this yielded in the large-scale development of the agriculture and the associated chemical industry, increase in resource consumption. Along with the accelerated industrial revolution recorded in the last two centuries, the societal evolution lead to serious and diverse environmental problems, such as rising levels of atmospheric carbon dioxide, global warming, soil and water pollution.
Chemical pollution of water is one of the major environmental problems in today’s world, since polluted water poses a threat to wildlife, as well as to human health and welfare, given that it hinders the sustainable development of both society and economy. Different contaminants are released into water bodies along with the rapid industrialization. Dye effluent from textile industry is one of the most important sources of environmental pollution. Approximately 10,000 different dyes and pigments (annual production of almost 7×105 tons) are utilized by textile industry. These dyes absorb the sunlight and reduce the photosynthetic capability of aquatic plants and microorganism, but, on the other hand, many of these dyes are toxic, carcinogenic, and mutagenic.
In recent years, treatment of waste water by physical and chemical methods has been the main focus in many studies. Advance Oxidation Processes (AOPs) have been effectively used to completely mineralize dyes present in textile waste water. Among these oxidation methods, photochemical catalysis appears as an emerging destructive technology leading to the total mineralization of many organic pollutants. A photocatalyst is a solid that can promote chemical reactions in the presence of light and is not consumed in the overall reaction. There is a list of demands that a photocatalyst should fulfil in order to be considered suitable for such application: it has to be able to utilize visible and/or UV light, photoactive, chemically and biologically inert, stable under irradiation, and non-toxic.
Several studies often highlighted the performance of ZnO in the degradation of some organic compounds, such as Remazol Brilliant Blue R, Remazol Black B, Reactive Blue 19 etc.. In addition, ZnO has more functions than TiO2 and the through research showed that ZnO can also be used in acidic or alkaline conditions.
Under the UV irradiation, holes and -OH radicals are formed on the surface of the photocatalyst, therefore, its degradation efficiency strongly depends on the fabrication method as it drives the shape and size of the photocatalyst particles (Figure 1).
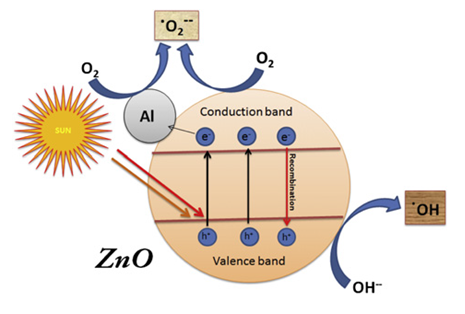
Figure 1. Photocatalysis mechanism of ZnO-Al-doped nanoparticles under solar light.
Efforts have been made by several research groups to improve the photocatalytic activity of ZnO by combining semiconductor substrates and metal clusters. For example, the efficiency of a photocatalytic reaction can be improved by incorporating noble metals on the TiO2 or ZnO particles. Research on ZnO has been carried out by various doping techniques such as chemical vapor deposition, metal organic chemical vapor deposition, the hydrothermal method, sol-gel deposition, and pulsed laser deposition (PLD) .

| Designed & Maintained by CLICK NET SOLUTIONS |



