|
Engineered eco-friendly biocomposites with selective chelating properties for removal and recovery of heavy metal ions from contaminated waters

|
| Home Team Members Project Abstract Objectives Budget Dissemination Contact |
Stage 2020: ![]()
Objective 3: Evaluation of ligand-functionalized polysaccharide-based composites as selective sorbents for removal and recovery of heavy metal ions from contaminated waters.
Objective 4: Modeling and optimization of polysaccharide modification, composite preparation and heavy metal ion sorption using tools of artificial intelligence.
Objective 5: Training/specialization of PhD and MS students, as well as the dissemination of project results.
Stage 3/2020 – Activities
A3.1. Investigation of selective removal/recovery of heavy metal ions under competitive conditions;
A3.2. Analysis of sorption mechanism and metal ion binding;
A3.3. Evaluation of composite regeneration and reusability in successive sorption/desorption cycles;
A3.4. Study of composite feasibility in fixed-bed columns, i.e. dynamic conditions;
A3.5. Modeling the ligand-containing composites preparation and sorption process using neural networks of different types;
A3.6. Optimization of polysaccharide modification, biocomposite preparation, and sorption process using genetic and differential evolution algorithms;
A3.7. Preparation and submission of manuscripts to ISI ranked journals;
A3.8. Update of the project web-page.
Stage 3/2020 – Results
- The modification in the amount of metal ions sorbed at equilibrium by polysaccharide-based composites cryogels induced by the pH variation was evaluated starting from five-component metal ion solutions with initial pH values ranging from 1.5 to 5.5 (Figure 1).
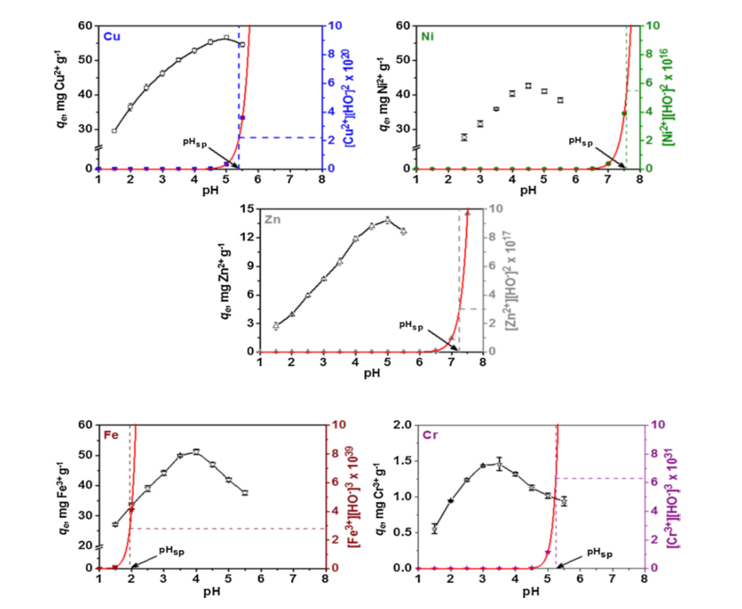
Figure 1. Effect of the initial solution pH on Cu2+, Ni2+, Zn2+, Fe3+, and Cr3+, removal by composites from five-component aqueous systems (pHsp is the pH of bulk solution precipitation) [Journal of Hazardous Materials 381 (2020) 120980; Doi:10.1016/j.jhazmat.2019.120980].
The maximum amount of Cu2+, Ni2+, Zn2+, and Cr3+, ions that was retained onto composites was obtained at pH values of 5, 4.5, 5, and 3.5 respectively, depending on the ion nature. The resulting pH of bulk solution precipitation (pHsp, Figure 1) indicates that the sorption of Cu2+, Ni2+, Zn2+, and Cr3+, ions is favored at pH values below pHsp, and thus demonstrates the absence of precipitation (with the exception of Fe3+, ions). In the case of Fe3+, ions, the increase of sorption capacity. even after the calculated pHsp value was reached, can be explained based on metal ion speciation, i.e. formation of FeOH2+ species that are thermodynamically more stable and easier to be adsorbed.
The difference in metal ion absorbency under the same conditions can be attributed to the different ionic features of these metal ions. In general, the relative selectivity of heavy metals is also related to some relevant metal properties, i.e. ionic radii, atomic weight, electronegativity, covalent index, ionic index, first hydrolysis constant, softness, and hydrated radius. The obtained sequences at pH 5 for our multi-component system (Cu2+>Ni2+>Fe3+>Zn2+>>Cr3+) are in a line only with the values of the covalent index. This parameter reflects the degree of covalent interactions in the metal–ligand complex relative to that of ionic interactions, suggesting the adsorption preference for Cu2+ over the other four metal ions.
- The handling of spent sorbents and the selective recovery of metal ions are two significant features which should be considered in development of economically feasible materials. In this study, the metal ions adsorbed on composites were successfully eluted with 0.1M HCl solution, regenerated with 0.1M NaOH solution, and reused in five consecutives sorption/desorption cycles (Figure 2).
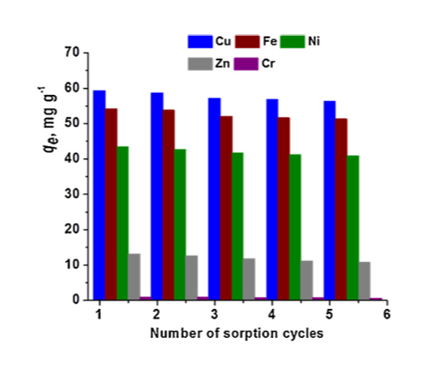
Figure 2. Influence of the sorption/desorption cycles on the sorption performance of the composite cryogel sorbent [Journal of Hazardous Materials 381 (2020) 120980; Doi:10.1016/j.jhazmat.2019.120980].
The composite sorbent exhibited a remarkable chemical stability during the loading/leaching steps of metal ions, the sorption capacity remaining almost constant after the 5th cycle (Figure 2).
Table 1. Selective sorption of Cu2+ ions onto IIC from its binary, ternary and five component mixtures with Zn2+, Ni2+, Fe3+, or Cr3+ ions [Journal of Hazardous Materials 381 (2020) 120980; Doi:10.1016/j.jhazmat.2019.120980].
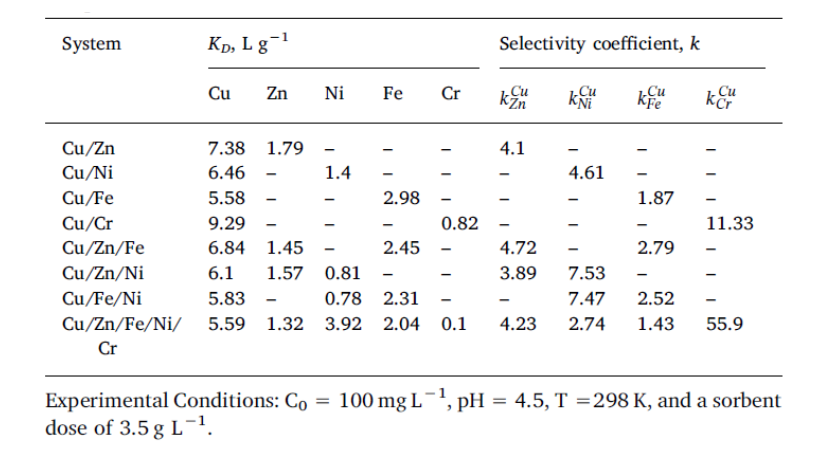
The composite sorbent presented higher KD and k values for Cu2+ ions compared to those calculated for Zn2+, Ni2+, Fe3+, or Cr3+ ions (Table 1), illustrating the major influence of ion-imprinting on the affinity and selectivity of engineered sorbent. This tendency was assigned to the coordination of metal ions mainly by the NH2 groups of chitosan during the first step of composite synthesis, before cryogelation, which led to well-defined imprinted cages with sizes controlled by the ionic radius of Cu2+.
- The batch sorption experiments showed a great potential of the CS-based composite sorbent in treating industrial wastewaters containing Cu2+, Zn2+, Ni2+, Fe3+, and Cr3+ ions. Therefore, its affinity against HMIs was also evaluated in dynamic conditions (Figure 3A).
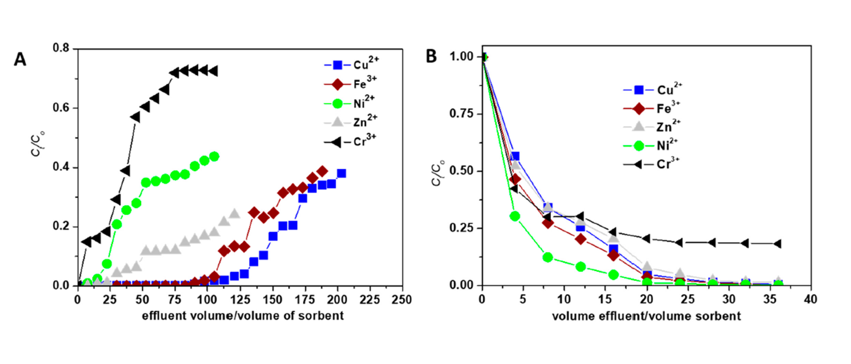
Figure 3. Sorption (A) and desorption (B) performance of CS-based composite sorbent in treating industrial wastewaters containing Cu2+, Zn2+, Ni2+, Fe3+, and Cr3+ ions under fixed-bed column conditions [Molecules 25 (2020) 2664; Doi:10.3390/molecules25112664].
- Co2+-imprinted composite cryo-beads with switching on/off selectivity towards the template ions, engineered by selecting the appropriate zeolite-treatment conditions and/or controlling the initial sorption pH values were successfully prepared. By comparing the uptake of Co2+ from their five-component mixtures using the ion-imprinted and non-imprinted composites, the high k values of Co2+-imprinted sorbents (Figure 4) pointed out towards the presence of recognition sites, which were properly introduced within the network structure of cryo-beads.
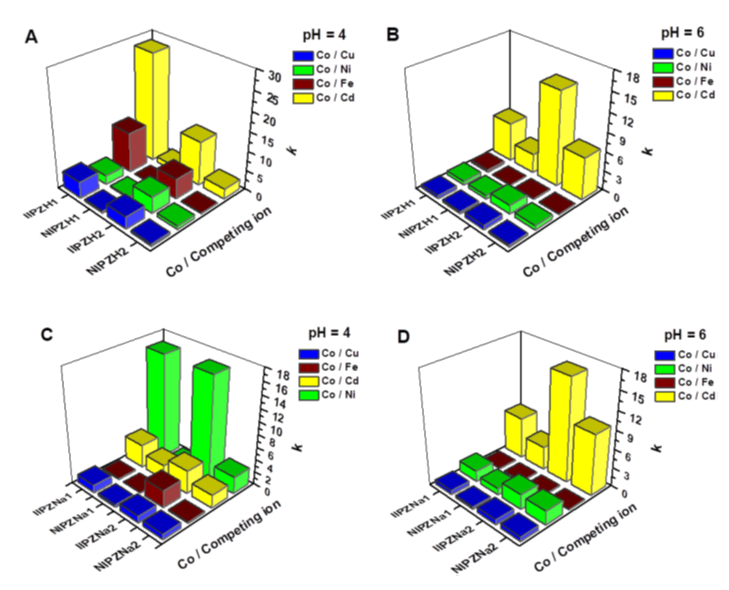
Figure 4. Selective sorption of Co2+ ions from their five-component mixtures, at pH 4 and 6, onto the composite cryo-beads containing zeolites activated with an aqueous solution of either 1 M HCl (A, B) or 1 M NaCl (C,D) [International Journal of Biological Macromolecules 164 (2020) 2432–2449; Doi:10.1016/j.ijbiomac.2020.08.009].
| Stage 2018 |
| Stage 2019 |
| Stage 2020 |

| Designed & Maintained by CLICK NET SOLUTIONS |



